HPV-Based Tests Warrant Longer Screening Intervals
|
By LabMedica International staff writers Posted on 04 Feb 2014 |

Image: Luminex Multiplexing System (Photo courtesy of Luminex).
The DNA test for human papillomavirus (HPV) allows for longer time between screening tests when compared to cytology-based testing.
Cervical screening programs have until recently relied on cytology to identify women at risk for developing cervical cancer, but it has long been known that screening with HPV DNA tests has a higher sensitivity for cervical intraepithelial neoplasia (CIN).
Scientists at the Karolinska Institutet (Stockholm, Sweden) enrolled 12,527 women aged 32 to 38 attending organized screening and randomized 6,257to HPV and cytology double testing, designated the intervention group, and 6,270 to cytology only, designated as the control group. Samples from the control group were frozen for future HPV testing. HPV DNA testing in the intervention group used polymerase chain reactions (PCR) with subsequent typing by reverse line dot blot hybridization. The types included in the HPV test were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
In the control group, the samples were frozen at −80 °C and retrieved for testing about 10 years later. HPV testing in the control group was based on the same testing principle but used improvements of the test that had been introduced in the meantime, namely, a switch to AmpliTaq Gold PCR enzyme (Life Technologies, Carlsbad, CA, USA) a modification of the general primers to increase sensitivity, and typing using Luminex technology (Austin, TX, USA).
At the follow-up 13 years after the start of the study, the scientists found that the increased detection rate for precancerous lesions of HPV-based screening reflects earlier detection rather than over-diagnosis. They also investigated the duration of the protective effect of the two screening methods by comparing overtime the incidence of precancerous lesions in women who had negative test results in the screening. They found that a negative test result in HPV screening is associated with a low risk for cervical intraepithelial neoplasia grade 2 or worse (CIN2+) or grade 3 or worse (CIN3+) and that this low risk is of prolonged duration.
The authors concluded that a five yearly screening interval for HPV negative women is an alternative to a three yearly screening interval for cytology negative women in those aged more than 30. The increased detection of CIN2 and CIN3+ during short-term follow-up of HPV based screening does not represent over diagnosis but rather early detection. This has important implications for the evaluation of the specificity and modelling of cost effectiveness of HPV based screening programs. The study was published on January 16, 2014, in the British Medical Journal (BMJ).
Related Links:
Karolinska Institutet
Life Technologies
Luminex
Cervical screening programs have until recently relied on cytology to identify women at risk for developing cervical cancer, but it has long been known that screening with HPV DNA tests has a higher sensitivity for cervical intraepithelial neoplasia (CIN).
Scientists at the Karolinska Institutet (Stockholm, Sweden) enrolled 12,527 women aged 32 to 38 attending organized screening and randomized 6,257to HPV and cytology double testing, designated the intervention group, and 6,270 to cytology only, designated as the control group. Samples from the control group were frozen for future HPV testing. HPV DNA testing in the intervention group used polymerase chain reactions (PCR) with subsequent typing by reverse line dot blot hybridization. The types included in the HPV test were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
In the control group, the samples were frozen at −80 °C and retrieved for testing about 10 years later. HPV testing in the control group was based on the same testing principle but used improvements of the test that had been introduced in the meantime, namely, a switch to AmpliTaq Gold PCR enzyme (Life Technologies, Carlsbad, CA, USA) a modification of the general primers to increase sensitivity, and typing using Luminex technology (Austin, TX, USA).
At the follow-up 13 years after the start of the study, the scientists found that the increased detection rate for precancerous lesions of HPV-based screening reflects earlier detection rather than over-diagnosis. They also investigated the duration of the protective effect of the two screening methods by comparing overtime the incidence of precancerous lesions in women who had negative test results in the screening. They found that a negative test result in HPV screening is associated with a low risk for cervical intraepithelial neoplasia grade 2 or worse (CIN2+) or grade 3 or worse (CIN3+) and that this low risk is of prolonged duration.
The authors concluded that a five yearly screening interval for HPV negative women is an alternative to a three yearly screening interval for cytology negative women in those aged more than 30. The increased detection of CIN2 and CIN3+ during short-term follow-up of HPV based screening does not represent over diagnosis but rather early detection. This has important implications for the evaluation of the specificity and modelling of cost effectiveness of HPV based screening programs. The study was published on January 16, 2014, in the British Medical Journal (BMJ).
Related Links:
Karolinska Institutet
Life Technologies
Luminex
Latest Microbiology News
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
- Rapid Diagnostic Test Matches Gold Standard for Sepsis Detection
- Rapid POC Tuberculosis Test Provides Results Within 15 Minutes
- Rapid Assay Identifies Bloodstream Infection Pathogens Directly from Patient Samples
- Blood-Based Molecular Signatures to Enable Rapid EPTB Diagnosis
- 15-Minute Blood Test Diagnoses Life-Threatening Infections in Children
- High-Throughput Enteric Panels Detect Multiple GI Bacterial Infections from Single Stool Swab Sample
- Fast Noninvasive Bedside Test Uses Sugar Fingerprint to Detect Fungal Infections
- Rapid Sepsis Diagnostic Device to Enable Personalized Critical Care for ICU Patients
- Microfluidic Platform Assesses Neutrophil Function in Sepsis Patients
Channels
Clinical Chemistry
view channel
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read more
Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
Chronic kidney disease (CKD) affects more than 1 in 7 Americans and is strongly associated with cardiovascular complications, which account for more than half of deaths among people with CKD.... Read moreMolecular Diagnostics
view channel
Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
Glioblastoma is one of the deadliest forms of brain cancer, largely because doctors have no reliable way to determine whether treatments are working in real time. Assessing therapeutic response currently... Read more
Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
Early-stage cancers are notoriously difficult to detect because molecular changes are subtle and often missed by existing screening tools. Many liquid biopsies rely on measuring absolute DNA methylation... Read more
“Lab-On-A-Disc” Device Paves Way for More Automated Liquid Biopsies
Extracellular vesicles (EVs) are tiny particles released by cells into the bloodstream that carry molecular information about a cell’s condition, including whether it is cancerous. However, EVs are highly... Read more
Blood Test Identifies Inflammatory Breast Cancer Patients at Increased Risk of Brain Metastasis
Brain metastasis is a frequent and devastating complication in patients with inflammatory breast cancer, an aggressive subtype with limited treatment options. Despite its high incidence, the biological... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more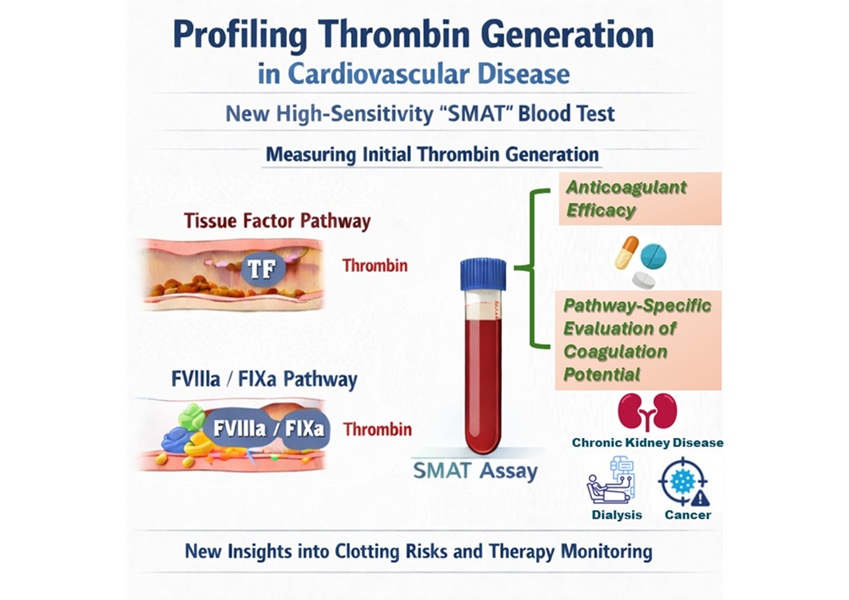
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read morePathology
view channel
Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Developing new cancer immunotherapies is a slow, costly, and high-risk process, particularly for CAR T cell treatments that must precisely recognize cancer-specific antigens. Small differences in tumor... Read more
First-Of-Its-Kind Test Identifies Autism Risk at Birth
Autism spectrum disorder is treatable, and extensive research shows that early intervention can significantly improve cognitive, social, and behavioral outcomes. Yet in the United States, the average age... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channelNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more
Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
Diasorin (Saluggia, Italy) has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific (Waltham, MA, USA), for the LIAISON NES molecular point-of-care... Read more