LumiraDx’s Broad Menu of Lab Comparable Results on Single, Portable Diagnostic Platform Promises to Transform Community Care
|
By LabMedica International staff writers Posted on 13 Nov 2021 |
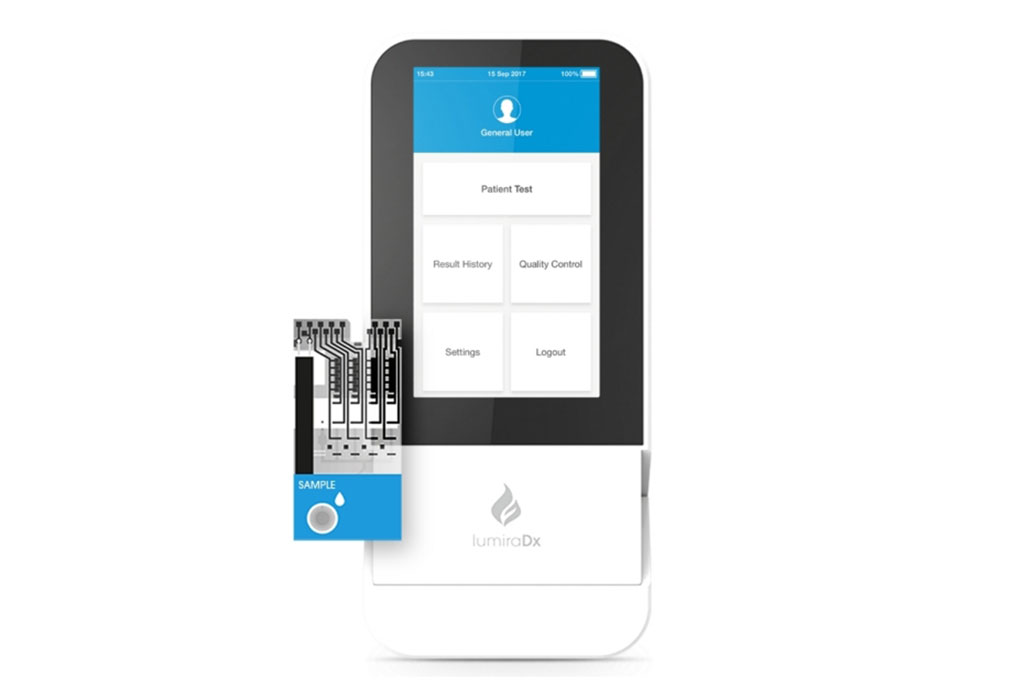
LumiraDx (London, UK) is offering a broad menu of lab comparable results on the LumiraDx Platform, its next generation point of care diagnostic system, that aims to transform community care.
The LumiraDx Platform combines a small, portable instrument, advanced low cost test strip and seamless secure digital connectivity to the Cloud and hospital IT systems. The LumiraDx Platform and tests are designed on the same principles as lab analyzer systems, to deliver accurate results compared to laboratory reference assays across a number of parameters, in a portable, easy-to-use point of care solution. The platform is designed to go wherever the patient is, whether this is in a hospital, medical office, pharmacy, or in other non-traditional settings such as schools or airports. Easy and intuitive to use, it requires only basic training and is designed to be affordable and accessible at the point of care. The company has a pipeline of more than 30 assays, initially focused on some of the most common conditions being diagnosed or managed with POC testing such as coagulation disorders, infectious diseases, cardiovascular and diabetes.
LumiraDx has developed SARS-CoV-2 antigen and antibody tests that provide lab comparable results for COVID-19 testing in minutes on a single platform. The LumiraDx SARS-CoV-2 Ag Test on the LumiraDx platform enables physicians to verify infection quickly, begin proper treatment and to initiate isolation precautions helping prevent further spread of infection. Similarly, the LumiraDx SARS-CoV-2 Ab Test is designed to be used in community care settings to identify individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. The company also offers the SARS-CoV-2 Ag Surveillance Test for environments such as schools and workplaces that allows simultaneous testing of up to five samples on its platform with cost per sample as low as USD 4. With digital results in less than 12 minutes from sample application and a comprehensive connectivity solution, it is designed to measure population health metrics in real-time to help prevent the spread of infection using the LumiraDx affordable, easy-to-use, onsite testing platform.
The LumiraDx Platform menu also includes point of care tests for INR and D-Dimer with high levels of accuracy comparable to central lab-based tests. The LumiraDx INR Test measures prothrombin time reported as International Normalized Ratio (INR) from a single, direct fingerstick blood sample - all in less than 90 seconds. The LumiraDx D-Dimer Test is an easy to use fluorescence immunoassay designed to rapidly quantify D-Dimer levels in human whole blood and plasma at the point of care. It is the first quantitative micro-fluidic immunoassay of its kind, providing a precise and accurate D-Dimer result from a single direct fingerstick blood sample, in only six minutes. All point of care tests on the LumiraDx Platform menu have achieved CE Mark and are commercially available in Europe.
Related Links:
LumiraDx
Latest COVID-19 News
- New Immunosensor Paves Way to Rapid POC Testing for COVID-19 and Emerging Infectious Diseases
- Long COVID Etiologies Found in Acute Infection Blood Samples
- Novel Device Detects COVID-19 Antibodies in Five Minutes
- CRISPR-Powered COVID-19 Test Detects SARS-CoV-2 in 30 Minutes Using Gene Scissors
- Gut Microbiome Dysbiosis Linked to COVID-19
- Novel SARS CoV-2 Rapid Antigen Test Validated for Diagnostic Accuracy
- New COVID + Flu + R.S.V. Test to Help Prepare for `Tripledemic`
- AI Takes Guesswork Out Of Lateral Flow Testing
- Fastest Ever SARS-CoV-2 Antigen Test Designed for Non-Invasive COVID-19 Testing in Any Setting
- Rapid Antigen Tests Detect Omicron, Delta SARS-CoV-2 Variants
- Health Care Professionals Showed Increased Interest in POC Technologies During Pandemic, Finds Study
- Set Up Reserve Lab Capacity Now for Faster Response to Next Pandemic, Say Researchers
- Blood Test Performed During Initial Infection Predicts Long COVID Risk
- Low-Cost COVID-19 Testing Platform Combines Sensitivity of PCR and Speed of Antigen Tests
- Finger-Prick Blood Test Identifies Immunity to COVID-19
- Quick Test Kit Determines Immunity Against COVID-19 and Its Variants
Channels
Clinical Chemistry
view channel
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read more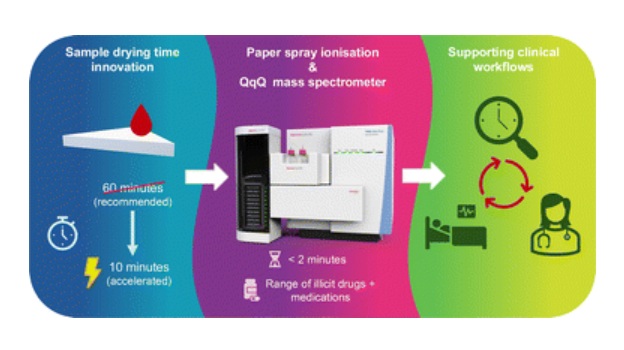
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read moreMolecular Diagnostics
view channel
New Extraction Kit Enables Consistent, Scalable cfDNA Isolation from Multiple Biofluids
Circulating cell-free DNA (cfDNA) found in plasma, serum, urine, and cerebrospinal fluid is typically present at low concentrations and is often highly fragmented, making efficient recovery challenging... Read more
AI-Powered Liquid Biopsy Classifies Pediatric Brain Tumors with High Accuracy
Liquid biopsies offer a noninvasive way to study cancer by analyzing circulating tumor DNA in body fluids. However, in pediatric brain tumors, the small amount of ctDNA in cerebrospinal fluid has limited... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Rapid Test Promises Faster Answers for Drug-Resistant Infections
Drug-resistant pathogens continue to pose a growing threat in healthcare facilities, where delayed detection can impede outbreak control and increase mortality. Candida auris is notoriously difficult to... Read more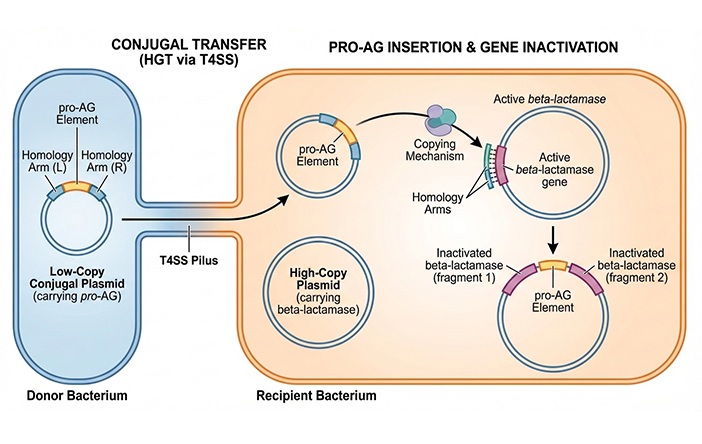
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, in part because of its dense tumor microenvironment that influences how tumors grow and respond to treatment.... Read more
New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
Sputum smear microscopy remains central to tuberculosis treatment monitoring and follow-up, particularly in high‑burden settings where serial testing is routine. Yet consistent, repeatable bacillary assessment... Read more
AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
Stem cell and bone marrow transplants can be lifesaving, but serious complications may arise months after patients leave the hospital. One of the most dangerous is chronic graft-versus-host disease, in... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreIndustry
view channel
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more