CRISPR-Powered COVID-19 Test Detects SARS-CoV-2 in 30 Minutes Using Gene Scissors
Posted on 29 Nov 2022
CRISPR-Cas is versatile: Besides the controversial genetically modified organisms (GMOs), created through gene editing, various new scientific studies use different orthologues of the effector protein ‘Cas’ to detect nucleic acids such as DNA or RNA. Now, a group of researchers has introduced a microfluidic multiplexed chip for the simultaneous measurement of the viral load in nasal swabs and (if applicable) the blood antibiotic levels of COVID-19 patients. The biosensor for the nucleic acid amplification-free detection of SARS-CoV-2 RNA was developed by researchers at University of Freiburg (Baden-Württemberg, Germany).
The market launch of rapid antigen test kits has significantly changed the way in which society handles the effects of the pandemic: Individuals suspecting an infection with SARS-CoV-2 can now test themselves at home with kits that are readily available at most drug stores, pharmacies and supermarkets, instead of making an, oftentimes difficult to acquire, appointment for PCR testing, that requires one to three additional days to receive the result. This convenience is, however, paid for with test sensitivity. This issue became flagrantly apparent during the wave of infections last winter, when the ‘lateral flow devices’ frequently failed to detect infections with the Omicron-variant until after the onset of symptoms.
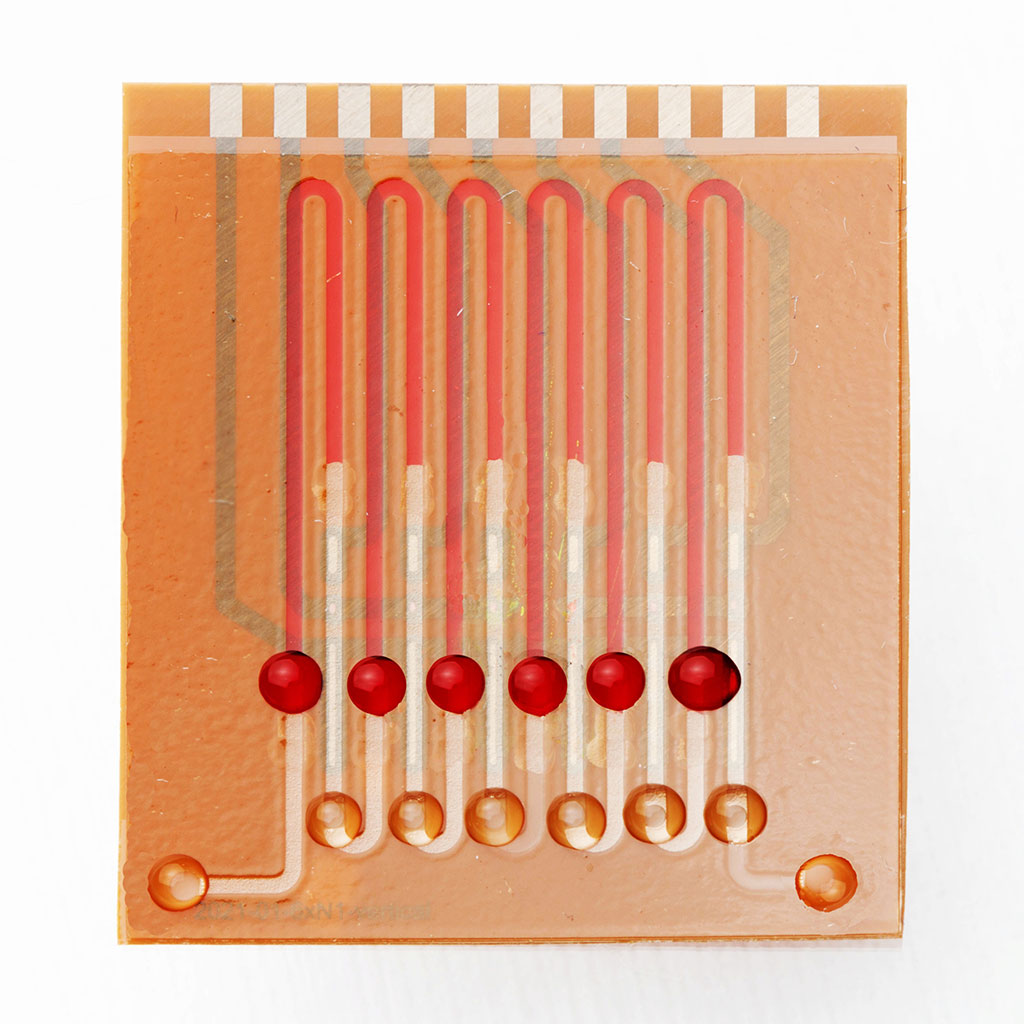
One alternative is a CRISPR-powered COVID-19 test. Similar to the rapid tests performed at home or in testing centers, a nasal or oral swab sample solution is added to a reaction mix. In contrast to these viral antigen tests, however, CRISPR, like rt-qPCR, screens the patient sample for RNA sequences characteristic to SARS-CoV-2. If the sample contains the RNA snippet of interest, the effector protein (Cas13a) is activated and cleaves the reporter RNA provided within the reaction mix. The absence of intact reporter creates an inversely proportional relation to the abundance of viral RNA within the sample, that is then analyzed in an electrochemical readout (low current densities indicate a high viral load).
In light of recent decisions of several federal states to discontinue isolation requirements for individuals tested positive for COVID-19 reliable, sensitive and rapid testing opportunities will again gain significance within the task of adequately managing recurring waves of infections. The latter will also inevitably coincide with the hospitalization of patients with severe symptoms and disease progressions. This is where another feature of the microfluidic chip comes into play: the combination of the CRISPR-assays with a ß-lactam antibiotic detection. COVID-19 patients often acquire bacterial co-infections that are then treated with broad-spectrum antibiotics like amoxicillin, ampicillin or piperacillin. Finding and maintaining the correct and personalized dosage is hereby crucial in guaranteeing a successful treatment as well as in reducing the emergence of resistant strains. The proposed sensor could facilitate dealing with these issues, through simultaneous monitoring of the viral load and blood antibiotic levels.
“The trade-off between sensitivity and sample-to-result time could potentially be bridged using our method,” said Midori Johnston, first author of the study.
“Our system herein omits nucleic acid amplification and is flexibly adaptable to new, clinically relevant mutations of the virus while exclusively employing inexpensive, shelf stable and non-toxic reagents as well as a portable measurement setup,” explained microsystems engineer Dr. Can Dincer of the Department of Microsystems Engineering, University of Freiburg who headed the research group.
Related Links:
University of Freiburg













