Immune Cell Biomarker Predicts Response to Treatment with Checkpoint Inhibitor Drugs
|
By LabMedica International staff writers Posted on 06 Sep 2021 |
![Image: Scanning electron micrograph of a human T-cell from the immune system of a healthy donor (Photo courtesy [U.S.] National Institute of Allergy and Infectious Diseases) Image: Scanning electron micrograph of a human T-cell from the immune system of a healthy donor (Photo courtesy [U.S.] National Institute of Allergy and Infectious Diseases)](https://globetechcdn.com/mobile_labmedica/images/stories/articles/article_images/2021-09-06/GMS-070B.jpg)
Image: Scanning electron micrograph of a human T-cell from the immune system of a healthy donor (Photo courtesy [U.S.] National Institute of Allergy and Infectious Diseases)
A molecular signature based on an immune cell surface protein is a potential route toward determining a cancer patient’s response to treatment with checkpoint inhibitor drugs.
Immune checkpoint inhibitors are drugs that can aid the immune system of some cancer patients to effectively attack their tumors, but identifying those patients for whom they will be effective is still a challenge.
In this regard, investigators at Memorial Sloan Kettering Cancer Center (New York, NY, USA) designed a study to identify blood-based biomarkers linked to the clinical outcome of immune checkpoint blockade (ICB)-treated patients. For this study, they performed immune profiling of 188 ICB-treated patients with melanoma using multiparametric flow cytometry to characterize immune cells in pretreatment peripheral blood. An independent cohort of 94 ICB-treated patients with urothelial carcinoma was used for validation.
The results revealed three distinct immune phenotypes (immunotypes), defined in part by the presence of a LAG-3+CD8+ T-cell population. LAG-3 (lymphocyte-activation gene 3) is a cell surface protein with diverse biologic effects on T-cell function, including an inhibitory effect on immune responses. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.
Patients with melanoma with a positive LAG immunotype had poorer outcomes after ICB with a median survival of 22.2 months compared to 75.8 months for those with the LAG negative immunotype. An independent cohort of 94 ICB-treated patients with urothelial carcinoma was used for validation where a LAG positive immunotype was significantly associated with response, survival, and progression-free survival.
"If I told you that you could have a simple blood draw and in a couple of days have information to make a decision about what therapy you get, I would say it does not get much better than that," said senior author Dr. Margaret K. Callahan, researcher in medical oncology at Memorial Sloan Kettering Cancer Center. "Of course, there is still much work to be done before these research findings can be applied to patients in the clinic, but we are really enthusiastic about the potential to apply these findings. What I am most excited about is prospectively evaluating the idea that not only can we identify patients who will not do as well with the traditional therapies but that we can also give these patients other treatments that might help them, based on our knowledge of what LAG-3 is doing biologically."
The LAG-3 study was published in the August 25, 2021, online edition of the journal Science Translational Medicine.
Related Links:
Memorial Sloan Kettering Cancer Center
Immune checkpoint inhibitors are drugs that can aid the immune system of some cancer patients to effectively attack their tumors, but identifying those patients for whom they will be effective is still a challenge.
In this regard, investigators at Memorial Sloan Kettering Cancer Center (New York, NY, USA) designed a study to identify blood-based biomarkers linked to the clinical outcome of immune checkpoint blockade (ICB)-treated patients. For this study, they performed immune profiling of 188 ICB-treated patients with melanoma using multiparametric flow cytometry to characterize immune cells in pretreatment peripheral blood. An independent cohort of 94 ICB-treated patients with urothelial carcinoma was used for validation.
The results revealed three distinct immune phenotypes (immunotypes), defined in part by the presence of a LAG-3+CD8+ T-cell population. LAG-3 (lymphocyte-activation gene 3) is a cell surface protein with diverse biologic effects on T-cell function, including an inhibitory effect on immune responses. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.
Patients with melanoma with a positive LAG immunotype had poorer outcomes after ICB with a median survival of 22.2 months compared to 75.8 months for those with the LAG negative immunotype. An independent cohort of 94 ICB-treated patients with urothelial carcinoma was used for validation where a LAG positive immunotype was significantly associated with response, survival, and progression-free survival.
"If I told you that you could have a simple blood draw and in a couple of days have information to make a decision about what therapy you get, I would say it does not get much better than that," said senior author Dr. Margaret K. Callahan, researcher in medical oncology at Memorial Sloan Kettering Cancer Center. "Of course, there is still much work to be done before these research findings can be applied to patients in the clinic, but we are really enthusiastic about the potential to apply these findings. What I am most excited about is prospectively evaluating the idea that not only can we identify patients who will not do as well with the traditional therapies but that we can also give these patients other treatments that might help them, based on our knowledge of what LAG-3 is doing biologically."
The LAG-3 study was published in the August 25, 2021, online edition of the journal Science Translational Medicine.
Related Links:
Memorial Sloan Kettering Cancer Center
Latest Molecular Diagnostics News
- New Testing Method Predicts Trauma Patient Recovery Days in Advance
- Simple Method Predicts Risk of Brain Tumor Recurrence
- Genetic Test Could Improve Early Detection of Prostate Cancer
- Bone Molecular Maps to Transform Early Osteoarthritis Detection
- POC Testing for Hepatitis B DNA as Effective as Traditional Laboratory Testing
- Fully Automated Immunoassay Test Detects HDV Co‑Infection and Super-Infection
- Abdominal Fluid Testing Can Predict Ovarian Cancer Progression
- POC Test Uses Fingerstick Blood, Serum, Or Plasma Sample to Detect Typhoid Fever
- Rapid Testing Panel Simultaneously Detects 15 Drugs of Abuse in Urine Within 21 Minutes
- New Test Detects Breast Reconstruction-Related Infections Before Symptoms Appear
- Period Blood Test for HPV Could Replace Cervical Screening
- New Genetic Tools Improve Breast Cancer Risk Prediction for African American Women
- Single-Use Test Strip to Revolutionize Disease Diagnosis
- Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
- Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
- Two-in-One DNA Analysis Improves Diagnostic Accuracy While Saving Time and Costs
Channels
Clinical Chemistry
view channel
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read more
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more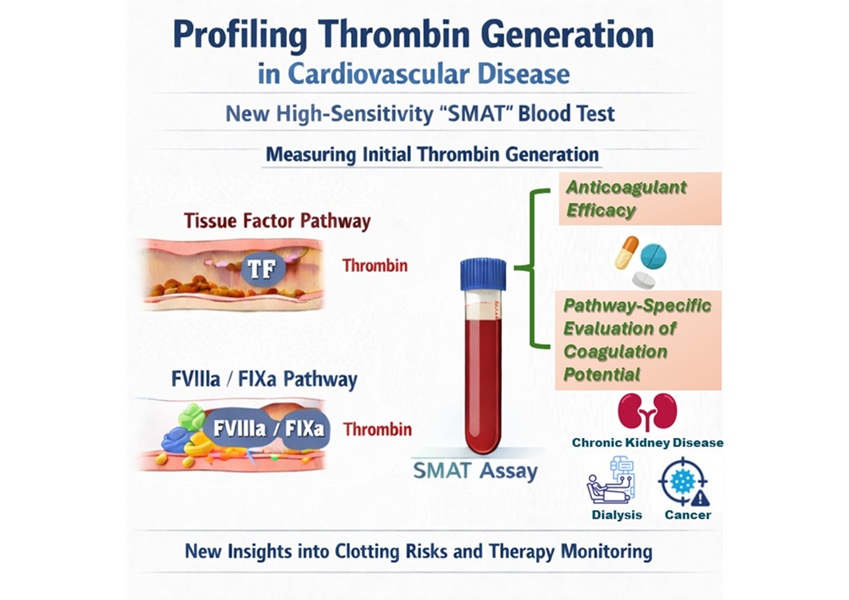
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
AI Tool Helps See How Cells Work Together Inside Diseased Tissue
Microscopes have long been central to diagnosing disease by allowing doctors to examine stained tissue samples. However, modern medical research now generates vast amounts of additional data, including... Read more
AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
Malaria remains one of the world’s deadliest infectious diseases, killing hundreds of thousands each year, mostly in under-resourced regions where laboratory infrastructure is limited. Diagnosis still... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channel
WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
WHX Labs in Dubai (formerly Medlab Middle East), held at Dubai World Trade Centre (DWTC) from 10–13 February, brings together international experts to discuss the factors redefining laboratory leadership,... Read moreNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more