Bone Molecular Maps to Transform Early Osteoarthritis Detection
Posted on 13 Feb 2026
Osteoarthritis affects more than 500 million people worldwide and is a major cause of pain, disability, and reduced quality of life. By the time it is diagnosed through symptoms and visible cartilage loss, the disease has often progressed significantly, and the damage is largely irreversible. Scientists have suspected that earlier changes may occur beneath the cartilage in subchondral bone, but the molecular basis of these changes has remained unclear. New research now suggests that molecular remodeling in bone may provide earlier and more reliable indicators of osteoarthritis progression, potentially opening the door to earlier diagnosis and intervention.
In a study led by the Buck Institute for Research on Aging (Novato, CA, USA), researchers analyzed human knee joint tissues from patients with end-stage osteoarthritis and compared them with non-OA controls using spatial matrix-assisted laser desorption/ionization mass spectrometry imaging combined with synovial fluid proteomics. This approach enabled high-resolution mapping of hundreds of proteins directly within bone and cartilage, rather than averaging signals across whole tissues.
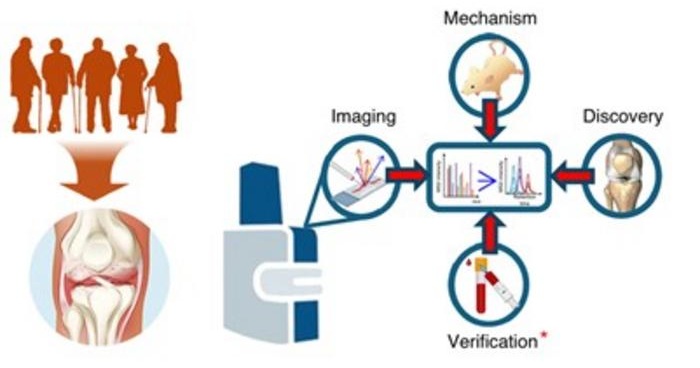
By enzymatically targeting extracellular matrix proteins, the team generated detailed molecular fingerprints that distinguished cartilage from bone and revealed localized remodeling patterns. The analysis showed strong upregulation of specific collagen fragments and post-translational modifications in subchondral bone beneath damaged cartilage.
Notably, similar molecular signatures were also detected in bone regions underlying cartilage that still appeared structurally intact, suggesting that bone remodeling begins earlier than visible cartilage degeneration. Many of these bone-derived protein fragments were also identified in synovial fluid, which can be obtained through minimally invasive procedures.
The findings, published in Bone Research, highlight subchondral bone remodeling as a potential source of early diagnostic biomarkers. The study indicates that bone remodeling, rather than cartilage breakdown alone, may offer more sensitive early signals of osteoarthritis. Traditional cartilage-associated markers were reduced in osteoarthritis joint fluid, further emphasizing the diagnostic potential of bone-specific molecular changes.
Researchers suggest that tracking these molecular signatures over time could help identify at-risk patients earlier and monitor therapeutic responses. Future work integrating spatial imaging, proteomics, and animal models aims to clarify how altered cellular activity in bone influences disease initiation and progression, supporting the development of targeted interventions before irreversible joint damage occurs.
“These results open the door to developing fluid-based tests that reflect what is happening deep within the joint,” said Dr. Charles A. Schurman, a postdoctoral research scientist. “If we can track bone-specific molecular changes over time, it may become possible to identify patients at risk earlier and monitor how they respond to therapy.”
Related Links:
Buck Institute for Research on Aging









 (3) (1).png)




