Researchers Deform Cells to Deliver RNA, Proteins, and Nanoparticles for Many Applications
|
By LabMedica International staff writers Posted on 06 Feb 2013 |
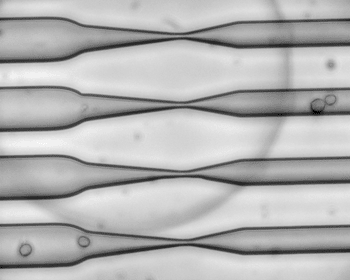
Image: As cells squeeze through a narrow channel, tiny holes open in their membranes, allowing large molecules such as RNA to pass through (Photo courtesy of Armon Sharei and Emily Jackson).
Researchers have found a safe and effective way to push large molecules through the cell membrane by jamming the cells through a narrow constriction that opens up very small, temporary holes in the membrane. Any large molecules drifting outside the cell—such as proteins, RNA, or nanoparticles—can slide through the membrane during this disruption.
Living cells are enclosed by a membrane that closely controls what gets in and out of the cell. This barrier is necessary for cells to control their internal environment, but it makes it more difficult for scientists to deliver large molecules such as nanoparticles for imaging, or proteins that can reprogram them into pluripotent stem cells.
Using this technique, the Massachusetts Institute of Technology (MIT; Cambridge, MA, USA: www.mit.edu) researchers were able to deliver reprogramming proteins and create induced pluripotent stem cells with a success rate 10 to 100 times superior than any existing application. They also used it to deliver nanoparticles, including quantum dots and carbon nanotubes, which can be used to image cells and track what is occurring inside them.
“It’s very useful to be able to get large molecules into cells. We thought it might be interesting if you could have a relatively simple system that could deliver many different compounds,” said Dr. Klavs Jensen, a professor of chemical engineering, professor of materials science and engineering, and a senior author of a paper describing the new device in this week’s issue of the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
Scientists had earlier developed several approaches to get large molecules into cells, but all of them have downsides. DNA or RNA can be parceled into viruses, which are proficient at entering cells, but that approach carries the risk that some of the viral DNA will be incorporated into the host cell. This application is commonly used in lab experiments but has not been approved by the US Food and Drug and Administration (FDA) for use in human patients.
Another way to transport large molecules into a cell is to tag them with a short protein that can penetrate the cell membrane and tug the larger payload along with it. Alternatively, DNA or proteins can be packaged into synthetic nanoparticles that can enter cells. However, these systems frequently need to be remodified depending on the type of cell and substance being delivered. Moreover, with some nanoparticles, a lot of the material ends up stuck in protective sacs called endosomes inside the cell, and there can be potential toxic side effects.
Electroporation, which involves jolting cells with electricity that opens up the cell membrane, is a more general approach but can be damaging to both cells and the material being delivered.
The new MIT system appears to work for many cell types—up to now, the researchers have successfully tested it with more than a dozen types, including both human and mouse cells. It also works in cells taken directly from human patients, which are typically much more difficult to engineer than human cell lines grown specifically for lab research.
The new device builds on earlier research by Jensen and Langer’s labs, in which they used microinjection to push large molecules into cells as they flowed through a microfluidic device. This was not as fast as the researchers hoped, but during these studies, they discovered that when a cell is squeezed through a narrow tube, small holes open in the cell membrane, allowing neighboring molecules to diffuse into the cell.
To take advantage of that, the researchers built rectangular microfluidic chips, about the size of a quarter, with 40 to 70 parallel channels. Cells are suspended in a solution with the material to be delivered and flowed through the channel at high speed—approximately one meter per second. Halfway through the channel, the cells pass through a constriction about 30%–80% smaller than the cells’ diameter. The cells do not sustain any permanent damage, and they maintain their normal functions after the treatment.
The scientists are now studying stem cell manipulation, which has potential for treating a wide range of diseases. They have already shown that they can convert human fibroblast cells into pluripotent stem cells, and now plan to start working on delivering the proteins needed to differentiate stem cells into specialized tissues.
Another promising application is delivering quantum dots—nanoparticles made of semiconducting metals that fluoresce. These dots hold promise for labeling individual proteins or other molecules inside cells, but scientists have had trouble getting them through the cell membrane without being trapped in endosomes.
In earlier work in November 2012, working with MIT graduate student Jungmin Lee and chemistry professor Dr. Moungi Bawendi, the researchers demonstrated that they could get quantum dots inside human cells grown in the laboratory, without the particles becoming confined in endosomes or clumping together. They are now working on getting the dots to tag specific proteins inside the cells.
The researchers are also exploring the possibility of using the new system for vaccination. In theory, scientists could take immune cells from a patient, run them through the microfluidic device and expose them to a viral protein, and then put them back in the patient. Once inside, the cells could provoke an immune response that would confer immunity against the target viral protein.
Related Links:
Massachusetts Institute of Technology
Living cells are enclosed by a membrane that closely controls what gets in and out of the cell. This barrier is necessary for cells to control their internal environment, but it makes it more difficult for scientists to deliver large molecules such as nanoparticles for imaging, or proteins that can reprogram them into pluripotent stem cells.
Using this technique, the Massachusetts Institute of Technology (MIT; Cambridge, MA, USA: www.mit.edu) researchers were able to deliver reprogramming proteins and create induced pluripotent stem cells with a success rate 10 to 100 times superior than any existing application. They also used it to deliver nanoparticles, including quantum dots and carbon nanotubes, which can be used to image cells and track what is occurring inside them.
“It’s very useful to be able to get large molecules into cells. We thought it might be interesting if you could have a relatively simple system that could deliver many different compounds,” said Dr. Klavs Jensen, a professor of chemical engineering, professor of materials science and engineering, and a senior author of a paper describing the new device in this week’s issue of the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
Scientists had earlier developed several approaches to get large molecules into cells, but all of them have downsides. DNA or RNA can be parceled into viruses, which are proficient at entering cells, but that approach carries the risk that some of the viral DNA will be incorporated into the host cell. This application is commonly used in lab experiments but has not been approved by the US Food and Drug and Administration (FDA) for use in human patients.
Another way to transport large molecules into a cell is to tag them with a short protein that can penetrate the cell membrane and tug the larger payload along with it. Alternatively, DNA or proteins can be packaged into synthetic nanoparticles that can enter cells. However, these systems frequently need to be remodified depending on the type of cell and substance being delivered. Moreover, with some nanoparticles, a lot of the material ends up stuck in protective sacs called endosomes inside the cell, and there can be potential toxic side effects.
Electroporation, which involves jolting cells with electricity that opens up the cell membrane, is a more general approach but can be damaging to both cells and the material being delivered.
The new MIT system appears to work for many cell types—up to now, the researchers have successfully tested it with more than a dozen types, including both human and mouse cells. It also works in cells taken directly from human patients, which are typically much more difficult to engineer than human cell lines grown specifically for lab research.
The new device builds on earlier research by Jensen and Langer’s labs, in which they used microinjection to push large molecules into cells as they flowed through a microfluidic device. This was not as fast as the researchers hoped, but during these studies, they discovered that when a cell is squeezed through a narrow tube, small holes open in the cell membrane, allowing neighboring molecules to diffuse into the cell.
To take advantage of that, the researchers built rectangular microfluidic chips, about the size of a quarter, with 40 to 70 parallel channels. Cells are suspended in a solution with the material to be delivered and flowed through the channel at high speed—approximately one meter per second. Halfway through the channel, the cells pass through a constriction about 30%–80% smaller than the cells’ diameter. The cells do not sustain any permanent damage, and they maintain their normal functions after the treatment.
The scientists are now studying stem cell manipulation, which has potential for treating a wide range of diseases. They have already shown that they can convert human fibroblast cells into pluripotent stem cells, and now plan to start working on delivering the proteins needed to differentiate stem cells into specialized tissues.
Another promising application is delivering quantum dots—nanoparticles made of semiconducting metals that fluoresce. These dots hold promise for labeling individual proteins or other molecules inside cells, but scientists have had trouble getting them through the cell membrane without being trapped in endosomes.
In earlier work in November 2012, working with MIT graduate student Jungmin Lee and chemistry professor Dr. Moungi Bawendi, the researchers demonstrated that they could get quantum dots inside human cells grown in the laboratory, without the particles becoming confined in endosomes or clumping together. They are now working on getting the dots to tag specific proteins inside the cells.
The researchers are also exploring the possibility of using the new system for vaccination. In theory, scientists could take immune cells from a patient, run them through the microfluidic device and expose them to a viral protein, and then put them back in the patient. Once inside, the cells could provoke an immune response that would confer immunity against the target viral protein.
Related Links:
Massachusetts Institute of Technology
Latest BioResearch News
- Genome Analysis Predicts Likelihood of Neurodisability in Oxygen-Deprived Newborns
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- Gene Fusion Protein Proposed as Prostate Cancer Biomarker
- NIV Test to Diagnose and Monitor Vascular Complications in Diabetes
- Semen Exosome MicroRNA Proves Biomarker for Prostate Cancer
- Genetic Loci Link Plasma Lipid Levels to CVD Risk
- Newly Identified Gene Network Aids in Early Diagnosis of Autism Spectrum Disorder
- Link Confirmed between Living in Poverty and Developing Diseases
- Genomic Study Identifies Kidney Disease Loci in Type I Diabetes Patients
- Liquid Biopsy More Effective for Analyzing Tumor Drug Resistance Mutations
- New Liquid Biopsy Assay Reveals Host-Pathogen Interactions
- Method Developed for Enriching Trophoblast Population in Samples
Channels
Clinical Chemistry
view channel
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read more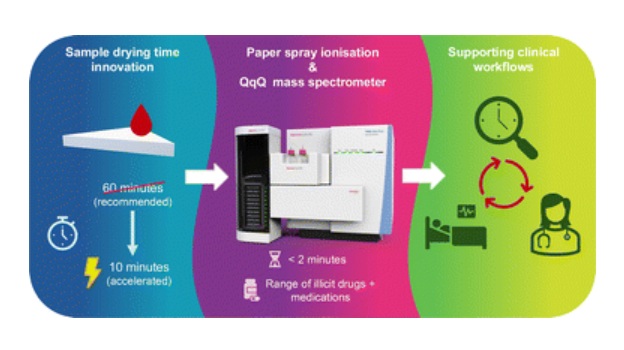
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read moreMolecular Diagnostics
view channel
New Extraction Kit Enables Consistent, Scalable cfDNA Isolation from Multiple Biofluids
Circulating cell-free DNA (cfDNA) found in plasma, serum, urine, and cerebrospinal fluid is typically present at low concentrations and is often highly fragmented, making efficient recovery challenging... Read more
AI-Powered Liquid Biopsy Classifies Pediatric Brain Tumors with High Accuracy
Liquid biopsies offer a noninvasive way to study cancer by analyzing circulating tumor DNA in body fluids. However, in pediatric brain tumors, the small amount of ctDNA in cerebrospinal fluid has limited... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Rapid Test Promises Faster Answers for Drug-Resistant Infections
Drug-resistant pathogens continue to pose a growing threat in healthcare facilities, where delayed detection can impede outbreak control and increase mortality. Candida auris is notoriously difficult to... Read more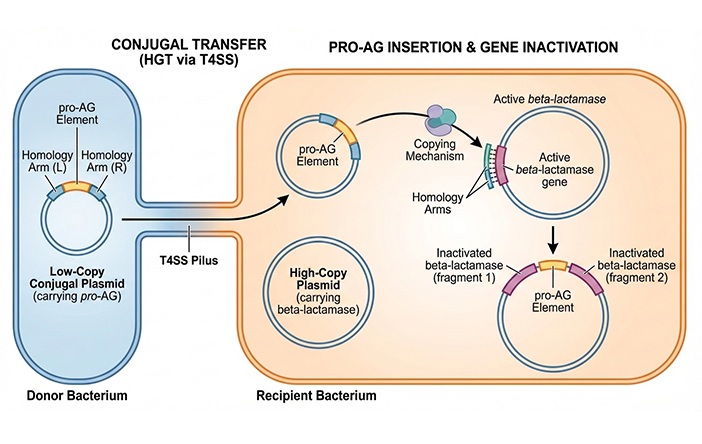
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, in part because of its dense tumor microenvironment that influences how tumors grow and respond to treatment.... Read more
New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
Sputum smear microscopy remains central to tuberculosis treatment monitoring and follow-up, particularly in high‑burden settings where serial testing is routine. Yet consistent, repeatable bacillary assessment... Read more
AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
Stem cell and bone marrow transplants can be lifesaving, but serious complications may arise months after patients leave the hospital. One of the most dangerous is chronic graft-versus-host disease, in... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreIndustry
view channel
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more