Cell-Free DNA Identifies Liver Transplant Patients with Acute Rejection
|
By LabMedica International staff writers Posted on 04 Aug 2016 |
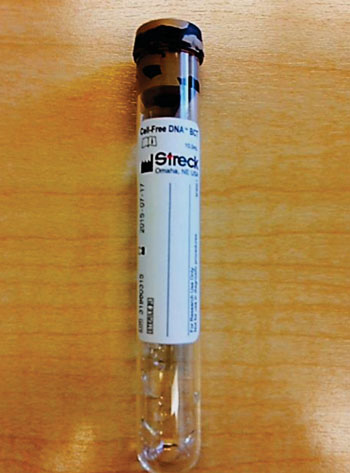
Image: A Cell-Free DNA BCT collection tube (Photo courtesy of Streck / Pathology Associates Medical Laboratory).
A cell-free DNA (cfDNA) test could help liver transplant patients receive crucial treatment for rejection faster, and has the potential to improve the prognosis of kidney and heart transplant patients as well.
Episodes of acute rejection, that is rejection that takes place in the first few months after an organ transplant, are relatively common. In liver transplant patients in particular, acute rejection develops in about 20% of those treated with standard immunosuppressive therapy. The gold standard for identifying rejection is biopsy, which is expensive and invasive, and at present there are no effective blood tests to take its place.
Scientists at Chronix Biomedical (Göttingen, Germany) and their associates determined whether a blood test for graft-derived cell-free DNA, which is cell-free DNA from a transplanted organ, could identify liver transplant patients with acute rejection. In a first-of-its-kind prospective multicenter trial, they monitored graft-derived cell-free DNA in the blood of 106 adult liver transplant recipients for at least one year post transplant. . Cell-free DNA was extracted from equal to or more than 1 mL EDTA plasma, obtained in Cell-free DNA-BCT tubes (Streck, Omaha, NE, USA). The turn-around time for an initial sample is about two days and one working day for any consecutive sample.
The teams found that in the 87 stable patients with no signs of graft injury and who were negative for hepatitis C virus infection, the median graft-derived cell-free DNA percentage decreased within the first week to a baseline level of less than 10% of total cell-free DNA concentrations. However, in the 20 patients with samples drawn during biopsy-proven acute rejection periods, graft-derived cell-free DNA levels were about 10-fold higher than those observed in the stable patients.
Overall they determined that by testing for graft-derived cell-free DNA levels of more than 10%, they were able to identify more than 90% of liver transplant patients with acute rejection, which was a substantially higher percentage than what conventional liver function tests can identify. They also believe that this test could detect heart and kidney transplant rejection, and are conducting additional studies to confirm this.
Ekkehard Schütz, MD, PhD, the senior author of the study, said, “This is really a universal test, you can use it for all kinds of solid organ transplantation since it’s just detecting the graft DNA, and it’s independent of what graft you are looking at. It will allow us to start treating these patients as early as possible, which not only impacts the acute situation that the patient is suffering at the time, but also impacts the long term survival of the graft. If we are able to diagnose rejection quickly enough, within a day or one and a half days, and the treating physician can react, then we can avoid really high-grade rejections further down the line.” The study was presented at the 68th American Association of Clinical Chemistry (AACC) Annual Scientific Meeting held July 31 to August 4, 2016, in Philadelphia, PA, USA.
Related Links:
Chronix Biomedical
Streck
American Association of Clinical Chemistry
Episodes of acute rejection, that is rejection that takes place in the first few months after an organ transplant, are relatively common. In liver transplant patients in particular, acute rejection develops in about 20% of those treated with standard immunosuppressive therapy. The gold standard for identifying rejection is biopsy, which is expensive and invasive, and at present there are no effective blood tests to take its place.
Scientists at Chronix Biomedical (Göttingen, Germany) and their associates determined whether a blood test for graft-derived cell-free DNA, which is cell-free DNA from a transplanted organ, could identify liver transplant patients with acute rejection. In a first-of-its-kind prospective multicenter trial, they monitored graft-derived cell-free DNA in the blood of 106 adult liver transplant recipients for at least one year post transplant. . Cell-free DNA was extracted from equal to or more than 1 mL EDTA plasma, obtained in Cell-free DNA-BCT tubes (Streck, Omaha, NE, USA). The turn-around time for an initial sample is about two days and one working day for any consecutive sample.
The teams found that in the 87 stable patients with no signs of graft injury and who were negative for hepatitis C virus infection, the median graft-derived cell-free DNA percentage decreased within the first week to a baseline level of less than 10% of total cell-free DNA concentrations. However, in the 20 patients with samples drawn during biopsy-proven acute rejection periods, graft-derived cell-free DNA levels were about 10-fold higher than those observed in the stable patients.
Overall they determined that by testing for graft-derived cell-free DNA levels of more than 10%, they were able to identify more than 90% of liver transplant patients with acute rejection, which was a substantially higher percentage than what conventional liver function tests can identify. They also believe that this test could detect heart and kidney transplant rejection, and are conducting additional studies to confirm this.
Ekkehard Schütz, MD, PhD, the senior author of the study, said, “This is really a universal test, you can use it for all kinds of solid organ transplantation since it’s just detecting the graft DNA, and it’s independent of what graft you are looking at. It will allow us to start treating these patients as early as possible, which not only impacts the acute situation that the patient is suffering at the time, but also impacts the long term survival of the graft. If we are able to diagnose rejection quickly enough, within a day or one and a half days, and the treating physician can react, then we can avoid really high-grade rejections further down the line.” The study was presented at the 68th American Association of Clinical Chemistry (AACC) Annual Scientific Meeting held July 31 to August 4, 2016, in Philadelphia, PA, USA.
Related Links:
Chronix Biomedical
Streck
American Association of Clinical Chemistry
Latest AACC 2016 News
- Molecular Test Detects Three Arboviruses in Plasma Samples
- Derived Exosomal Protein Biomarkers in Alzheimer’s Disease Diagnosis
- New Biochip Array Developed for ApoE4 Classification
- New Method Tested for Early Diagnosis Pediatric Diabetic Nephropathy
- FDA-Cleared Automated Cell Counter for CSF Launched at AACC 2016
- Semen Analysis Portfolio with Two New Products Featured at AACC 2016
- Automation Solutions for Clinical Diagnostic Equipment Showcased at AACC 2016
- New Tubes Designed for Medium Sample Volumes
- Multi Sample Osmometer Improves Testing Efficiency
- Innovative Information System Optimizes Laboratory Processes
- Innovative eLearning Interface Seamlessly Connects Competency Data
- Cloud-Based Connectivity Platform Advances Decentralized Healthcare
- Adhesives Research to Present Hydrophilic Adhesive Technologies
- Point-of-Care Immunoassay Analyzer on Display at AACC Annual Meeting
- Assay for Determination of 17-OH Progesterone to Be Featured at AACC Annual Meeting
- Fully Automated HbA1c Analyzer Available for Inspection at AACC Annual Meeting
Channels
Clinical Chemistry
view channel
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read more
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read moreMolecular Diagnostics
view channel
Light-Based Sensor Detects Early Molecular Signs of Cancer in Blood
Early cancer diagnosis is often hindered by the extremely low concentration of biomarkers present at the onset of disease. Proteins, DNA fragments, and other molecular markers can reveal cancer risk or... Read more
New Testing Method Predicts Trauma Patient Recovery Days in Advance
Trauma patients with nearly identical injuries often experience very different recoveries, even when treated similarly. Traditional assessments based on injury severity do not always explain why some patients... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more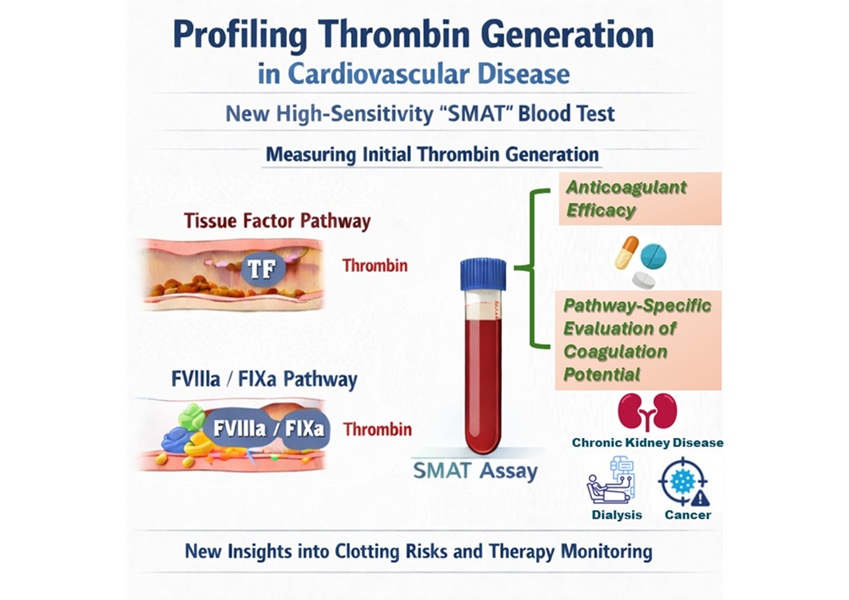
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
AI Tool Helps See How Cells Work Together Inside Diseased Tissue
Microscopes have long been central to diagnosing disease by allowing doctors to examine stained tissue samples. However, modern medical research now generates vast amounts of additional data, including... Read more
AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
Malaria remains one of the world’s deadliest infectious diseases, killing hundreds of thousands each year, mostly in under-resourced regions where laboratory infrastructure is limited. Diagnosis still... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channel
WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
WHX Labs in Dubai (formerly Medlab Middle East), held at Dubai World Trade Centre (DWTC) from 10–13 February, brings together international experts to discuss the factors redefining laboratory leadership,... Read moreNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more









 (3) (1).png)