Semen Analysis Portfolio with Two New Products Featured at AACC 2016
By LabMedica International staff writers
Posted on 04 Aug 2016
Showcasing at the AACC Annual Meeting & Clinical Lab Expo (July 31 - August 4; Philadelphia, PA, USA) is a state-of-the-art automated semen analysis system and a wide variety of sperm evaluation kits and tools for manual as well as computer-aided semen analysis.Posted on 04 Aug 2016
Medical Electronic Systems (MES; Los Angeles, CA, USA, and Caesarea, Israel) will be showcasing their SQA-VISION as well as two newly released products – QwikCheck Pre-Stained Morphology slides and a Linearity and Precision kit – that supplement MES’s comprehensive line of semen analysis solutions.
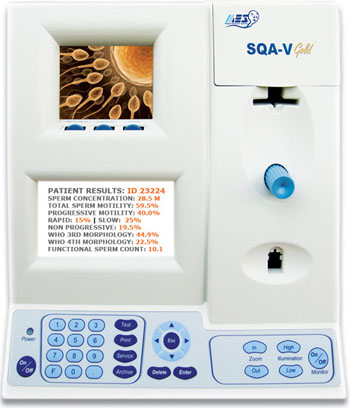
Image: The SQA-V Gold with V-Sperm Gold (Photo courtesy of Medical Electronic Systems).
The QwikCheck Pre-Stained Morphology slides are designed for rapid staining of spermatozoa for detailed morphology assessment. Each slide is coated with a dried layer of staining media that sperm will absorb on contact. The product is designed for SQA-VISION Automated Sperm Quality Analyzers and microscopic analysis. They can be used to differentiate normal sperm cells from sperm cells with abnormal heads, midpieces, principle pieces, and excess residual cytoplasm. They can also be used for the classification of WBCs and pinheads.
The QwikCheck Beads Precision and Linearity Kit is designed as a semi-annual validation tool for SQA-V and SQA-VISION sperm quality analyzers. It can be used to validate linearity, precision, and concentration accuracy per the CLIA Method Validation Regulations (CLIA Final Rules Manual, 2004, ISBN 1-886958-20-3).
Related Links:
Medical Electronic Systems