FDA-Cleared Automated Cell Counter for CSF Launched at AACC 2016
By LabMedica International staff writers
Posted on 04 Aug 2016
The system provides superior accuracy of low cell counts in cerebrospinal fluid samples. It is being introduced at the AACC Annual Scientific Meeting & Clinical Lab Expo (July 31 - August 4; Philadelphia, PA, USA). Linda Sandhaus, MD, University Hospitals Case Medical Center, presented a poster (#A-290) on August 2 titled GloCyte: A New Automated Technology for Cerebrospinal Fluid (CSF) Cell Counts.Posted on 04 Aug 2016
Advanced Instruments, Inc. (Norwood, MA, USA) has received 510(k) clearance from the US Food & Drug Administration (FDA) to market its GloCyte Automated Cell Counter System and GloCyte Low and High Level Controls. The patented GloCyte System is intended to provide a quantitative determination of red blood cells (RBCs) and total nucleated cells (TNCs) in CSF collected from adult and pediatric patients. Currently, low cell counts often present a challenge to standard methods.
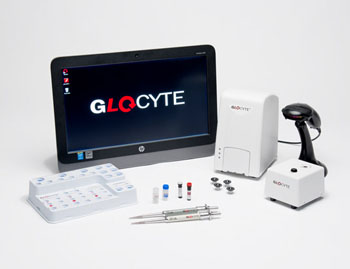
Image: The GloCyte system delivers highly accurate and precise total nucleated cell and red blood cell counts using a novel combination of technologies (Photo courtesy of Advanced Instruments).
“To date, there has not been a way to provide dependable, low cell counts,” said John Coughlin, president and CEO, Advanced Instruments, “We use a novel combination of fluorescence, microscopy with digital image analysis principles, highly specific reagents, and an intelligent counting algorithm to provide accurate and precise cell counts. We are very excited as this marks a major achievement in giving laboratories a new way to obtain reliable and timely CSF results.”
Additional benefits of the GloCyte System are that the test requires only 30 microliters of sample per test, it uses disposable test cartridges ensuring no sample carryover and easy disposal, and it includes built-in quality control of Levey-Jennings charts and an audit table. The company expects to begin GloCyte shipments in September 2016.
Related Links:
Advanced Instruments










 (3) (1).png)