Cloud-Based Connectivity Platform Advances Decentralized Healthcare
By LabMedica International staff writers
Posted on 03 Aug 2016
A novel reporting platform enables real-time oversight, fragile network connectivity, and actionable insights for medical device manufacturers and healthcare providers.Posted on 03 Aug 2016
The Fio (Toronto, Canada) Fionet platform connects mobile companion devices that guide diagnosis, treatment, and record keeping with web-based tools for remote oversight and reporting. Fionet mobile software is designed for standard Android devices and the proprietary Deki Reader, a rugged, in vitro diagnostic (IVD) device for use with commercially available lateral flow immunoassays. As the Deki Reader provides step-by-step guidance for performing rapid diagnostic tests and delivers an objective analysis of results, Fionet provides test-by-test traceability.
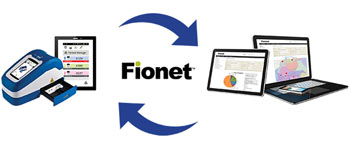
Image: Fionet connectivity with the Fio Deki Reader (Photo courtesy of Fio).
As connectivity becomes an essential prerequisite for diagnostic platforms, Fionet meets the challenge by providing web-based tools for timely, accurate and complete record taking of individual patient visits; generating reports from aggregated, primary data; allowing two-way messaging with companion IVD devices; and easy data transfer and information technology (IT) integration. Fio will present the Fionet platform at AACC 2016.
“With healthcare services becoming more and more decentralized, device manufacturers are adapting their products from the lab outward. At Fio, we’ve built our data services from the field inward,” said Ian Fine, Chief Technology Officer of Fio. “Fio’s appearance at the AACC trade fair this year will provide an opportunity for the company to share insights from connecting diagnostics and data in developing countries.”
The American Association for Clinical Chemistry (AACC; Washington, DC, USA) is an international scientific/medical society of over 8,000 clinical laboratory professionals, physicians, research scientists and more. The AACC annual meeting, which will be held from July 31st to August 4th at the Pennsylvania Convention Center in Philadelphia (USA), features five days of educational sessions on scientific, clinical, technical and management challenges facing laboratory professionals, as well as the world’s largest Clinical Lab Expo.
Related Links:
Fio
American Association for Clinical Chemistry