Bio-Rad ddPCR COVID-19 Assay Secures FDA Emergency Use Authorization
|
By LabMedica International staff writers Posted on 05 May 2020 |

Image: QX200 AutoDG Droplet Digital PCR (Photo courtesy of Bio-Rad Laboratories, Inc.)
Bio-Rad Laboratories, Inc.’s (Hercules, CA, USA) SARS-CoV-2 Droplet Digital PCR (ddPCR) test kit which runs on its QX200 and QXDx ddPCR systems has been granted Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA).
The SARS-CoV-2 ddPCR test represents the latest addition to Bio-Rad’s product offerings related to COVID-19 and is the second EUA that has been granted to the company for COVID-19. Last week, Bio-Rad had secured EUA for its serology test to detect antibodies to the virus.
The high sensitivity of Bio-Rad’s single-well SARS-CoV-2 ddPCR test makes it well suited to screening upper respiratory samples in patients with a low viral load, including individuals in the early stages of infection as compared to classical quantitative PCR tests. The test can also play an important role in surveillance by detecting minimal residual disease in people recovering from COVID-19 and informing them if they are negative for the virus. It also provides clinicians with a high degree of sensitivity that can significantly improve the accuracy of reported results. Thousands of hospitals and molecular testing labs in the US and around the world have installed Bio-Rad’s QX200 and QXDx ddPCR systems and have the capacity to perform SARS-CoV-2 ddPCR testing.
“We are pleased to receive Emergency Use Authorization for our SARS-CoV-2 ddPCR test that offers high sensitivity in detecting infection of COVID-19 at an early stage,” said Simon May, Senior Vice President of Bio-Rad’s Digital Biology Group. “We believe the test will be a powerful complement to current diagnostics, helping clinicians and public health officials accurately identify infected individuals in a timely manner. With the EUA, our test is now available to clinical labs in the US.”
The SARS-CoV-2 ddPCR test represents the latest addition to Bio-Rad’s product offerings related to COVID-19 and is the second EUA that has been granted to the company for COVID-19. Last week, Bio-Rad had secured EUA for its serology test to detect antibodies to the virus.
The high sensitivity of Bio-Rad’s single-well SARS-CoV-2 ddPCR test makes it well suited to screening upper respiratory samples in patients with a low viral load, including individuals in the early stages of infection as compared to classical quantitative PCR tests. The test can also play an important role in surveillance by detecting minimal residual disease in people recovering from COVID-19 and informing them if they are negative for the virus. It also provides clinicians with a high degree of sensitivity that can significantly improve the accuracy of reported results. Thousands of hospitals and molecular testing labs in the US and around the world have installed Bio-Rad’s QX200 and QXDx ddPCR systems and have the capacity to perform SARS-CoV-2 ddPCR testing.
“We are pleased to receive Emergency Use Authorization for our SARS-CoV-2 ddPCR test that offers high sensitivity in detecting infection of COVID-19 at an early stage,” said Simon May, Senior Vice President of Bio-Rad’s Digital Biology Group. “We believe the test will be a powerful complement to current diagnostics, helping clinicians and public health officials accurately identify infected individuals in a timely manner. With the EUA, our test is now available to clinical labs in the US.”
Latest COVID-19 News
- New Immunosensor Paves Way to Rapid POC Testing for COVID-19 and Emerging Infectious Diseases
- Long COVID Etiologies Found in Acute Infection Blood Samples
- Novel Device Detects COVID-19 Antibodies in Five Minutes
- CRISPR-Powered COVID-19 Test Detects SARS-CoV-2 in 30 Minutes Using Gene Scissors
- Gut Microbiome Dysbiosis Linked to COVID-19
- Novel SARS CoV-2 Rapid Antigen Test Validated for Diagnostic Accuracy
- New COVID + Flu + R.S.V. Test to Help Prepare for `Tripledemic`
- AI Takes Guesswork Out Of Lateral Flow Testing
- Fastest Ever SARS-CoV-2 Antigen Test Designed for Non-Invasive COVID-19 Testing in Any Setting
- Rapid Antigen Tests Detect Omicron, Delta SARS-CoV-2 Variants
- Health Care Professionals Showed Increased Interest in POC Technologies During Pandemic, Finds Study
- Set Up Reserve Lab Capacity Now for Faster Response to Next Pandemic, Say Researchers
- Blood Test Performed During Initial Infection Predicts Long COVID Risk
- Low-Cost COVID-19 Testing Platform Combines Sensitivity of PCR and Speed of Antigen Tests
- Finger-Prick Blood Test Identifies Immunity to COVID-19
- Quick Test Kit Determines Immunity Against COVID-19 and Its Variants
Channels
Clinical Chemistry
view channel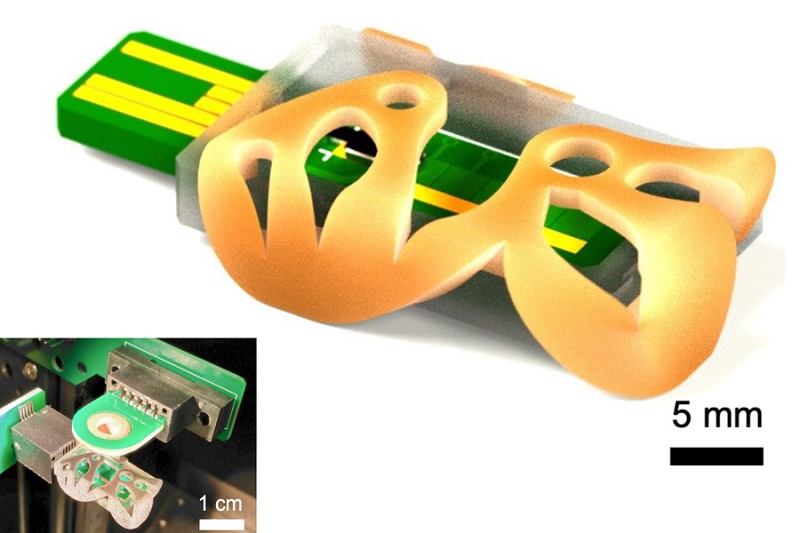
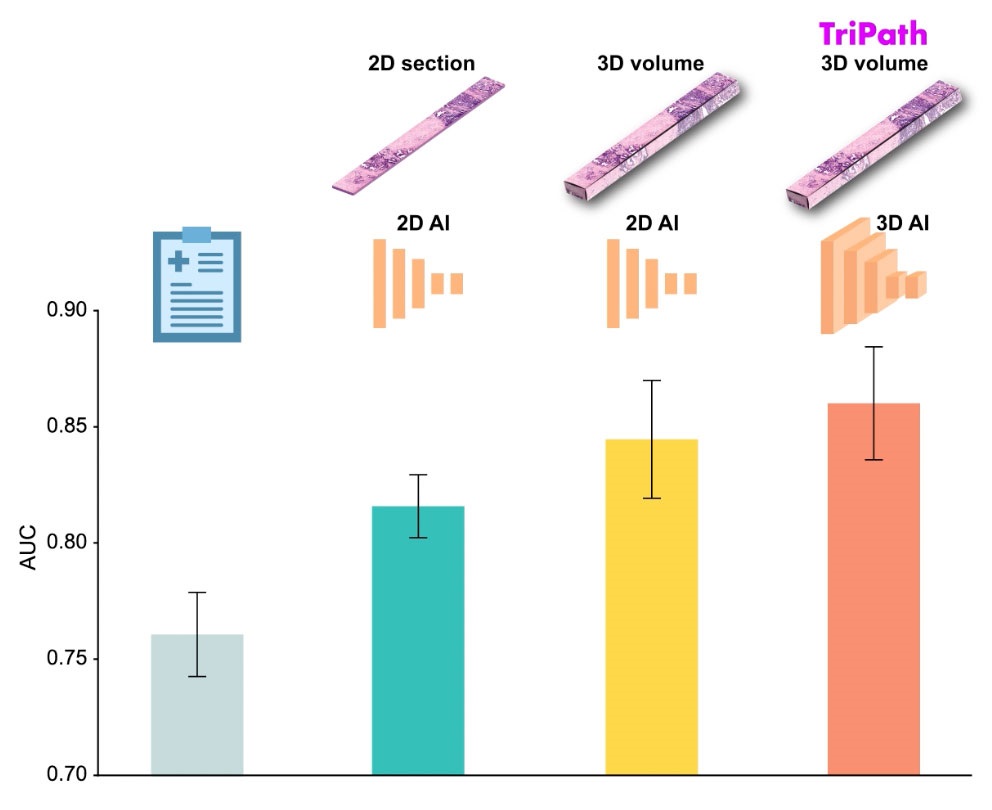
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more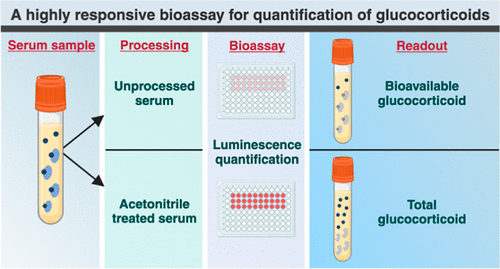
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channelBlood Proteins Could Warn of Cancer Seven Years before Diagnosis
Two studies have identified proteins in the blood that could potentially alert individuals to the presence of cancer more than seven years before the disease is clinically diagnosed. Researchers found... Read moreUltrasound-Aided Blood Testing Detects Cancer Biomarkers from Cells
Ultrasound imaging serves as a noninvasive method to locate and monitor cancerous tumors effectively. However, crucial details about the cancer, such as the specific types of cells and genetic mutations... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel.jpg)
AI Predicts Tumor-Killing Cells with High Accuracy
Cellular immunotherapy involves extracting immune cells from a patient's tumor, potentially enhancing their cancer-fighting capabilities through engineering, and then expanding and reintroducing them into the body.... Read more
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channel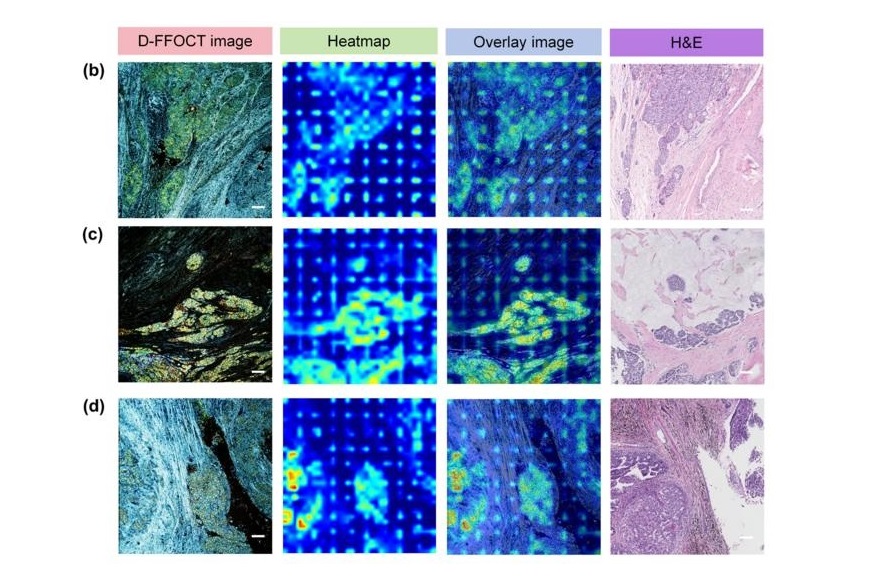
AI Integrated With Optical Imaging Technology Enables Rapid Intraoperative Diagnosis
Rapid and accurate intraoperative diagnosis is essential for tumor surgery as it guides surgical decisions with precision. Traditional intraoperative assessments, such as frozen sections based on H&E... Read more
HPV Self-Collection Solution Improves Access to Cervical Cancer Testing
Annually, over 604,000 women across the world are diagnosed with cervical cancer, and about 342,000 die from this disease, which is preventable and primarily caused by the Human Papillomavirus (HPV).... Read moreHyperspectral Dark-Field Microscopy Enables Rapid and Accurate Identification of Cancerous Tissues
Breast cancer remains a major cause of cancer-related mortality among women. Breast-conserving surgery (BCS), also known as lumpectomy, is the removal of the cancerous lump and a small margin of surrounding tissue.... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more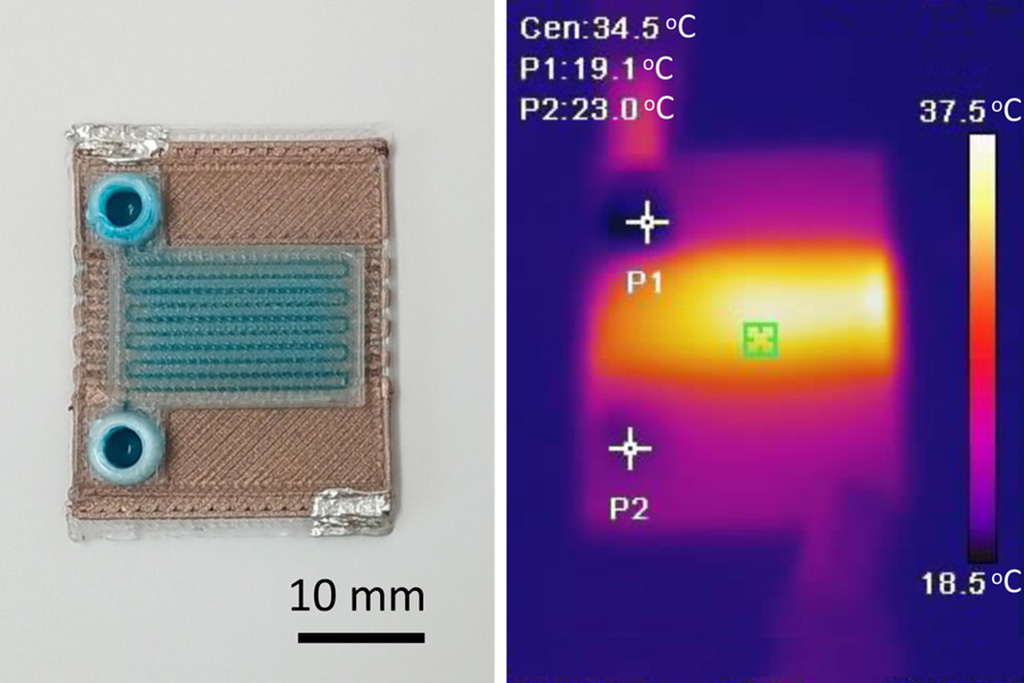
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
Unlike severe traumatic brain injury (TBI), mild TBI often does not show clear correlations with abnormalities detected through head computed tomography (CT) scans. Consequently, there is a pressing need... Read more
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)













_1.jpg)
.jpg)