Urine Testing Method Developed for Ureterolithiasis
|
By LabMedica International staff writers Posted on 03 Jun 2020 |

Image: FlexStation 3 Microplate Reader measures absorbance, fluorescence intensity, fluorescence polarization, luminescence, and time-resolved fluorescence (Photo courtesy of Molecular Devices).
Ureterolithiasis (nephrolithiasis or kidney stones) is a disease affecting the urinary tract. Kidney stones occur due to buildup of certain salts and minerals that form crystals, which in turn stick together and enlarge to form a hard mass in the kidneys. The stones move into the urinary tract and can cause blood in the urine, considerable pain and blockages in the urinary system.
Metabolic testing of a kidney stone patient’s urine to identify metabolites such as minerals and solutes that cause stones to form is key for preventing future ones. This testing is currently done by requiring the patient to collect their urine over a 24-hour period in a large container. The container is then sent to a laboratory for analysis and the results normally come back in 7 to 10 days.
Scientists from the Pennsylvania State University (University Park, PA, USA) and their colleagues developed SLIPS-LAB (Slippery Liquid-Infused Porous Surface Laboratory), a droplet-based bioanalysis system, for rapid measurement of urinary stone–associated analytes. The ultra-repellent and antifouling properties of SLIPS, which is a biologically inspired surface technology, allow autonomous liquid handling and manipulation of physiological samples without complicated sample preparation procedures and supporting equipment.
The team used enzymatic and colorimetric assay kits to measure metabolic profiles in urine. The uric acid assay was obtained from BioAssay Systems (Hayward CA, USA). Calibration experiments were performed using SLIPS-LAB and a FlexStation 3 microplate reader (Molecular Devices, San Jose, CA, USA). The performance of SLIPS-LAB for a spot urine test was compared with manual procedures in 96-well plates. A validation study was performed using SLIPS-LAB for detecting urine samples from patients with urinary stones.
The six-plex SLIPS-LAB device was designed for metabolic evaluation of urinary stone disease. The device conducts colorimetric and enzymatic assays in parallel for detecting calcium, citrate, uric acid, oxalate, and pH, which are among the most clinically relevant urinary analytes to assess stone risk and treatment response. The urinary stone–associated analytes of 24-hour urine samples from 15 individuals were examined using SLIPS-LAB. To study the metabolic profiles, the data were normalized to typical physiological values of 24-hour urine.
The test results can then be read using a scanner or a cell phone, and the scanned image can then be analyzed using a computer algorithm. All these steps, according to the authors, would take approximately 30 minutes in a physician’s office. An added benefit, they said, is that SLIPS-LAB is more cost-effective than regular, 24-hour testing.
Pak Kin Wong, PhD, professor of biomedical engineering and the principal investigator of the study, said, “We demonstrated that SLIPS-LAB enables the reagent and sample to move themselves and perform the reactions for us. It means the technology doesn’t require a technician to run any test machinery, so it is possible to do the test in non-traditional settings, like a physician’s office or even the patient’s home.”
The authors concluded that SLIPS-LAB will allow detection in a timely and cost-efficient manner and provide actionable diagnostic information and personalized treatment suggestions to individuals with urinary stone disease. The study was published on May 22, 2020 in the journal Science Advances.
Metabolic testing of a kidney stone patient’s urine to identify metabolites such as minerals and solutes that cause stones to form is key for preventing future ones. This testing is currently done by requiring the patient to collect their urine over a 24-hour period in a large container. The container is then sent to a laboratory for analysis and the results normally come back in 7 to 10 days.
Scientists from the Pennsylvania State University (University Park, PA, USA) and their colleagues developed SLIPS-LAB (Slippery Liquid-Infused Porous Surface Laboratory), a droplet-based bioanalysis system, for rapid measurement of urinary stone–associated analytes. The ultra-repellent and antifouling properties of SLIPS, which is a biologically inspired surface technology, allow autonomous liquid handling and manipulation of physiological samples without complicated sample preparation procedures and supporting equipment.
The team used enzymatic and colorimetric assay kits to measure metabolic profiles in urine. The uric acid assay was obtained from BioAssay Systems (Hayward CA, USA). Calibration experiments were performed using SLIPS-LAB and a FlexStation 3 microplate reader (Molecular Devices, San Jose, CA, USA). The performance of SLIPS-LAB for a spot urine test was compared with manual procedures in 96-well plates. A validation study was performed using SLIPS-LAB for detecting urine samples from patients with urinary stones.
The six-plex SLIPS-LAB device was designed for metabolic evaluation of urinary stone disease. The device conducts colorimetric and enzymatic assays in parallel for detecting calcium, citrate, uric acid, oxalate, and pH, which are among the most clinically relevant urinary analytes to assess stone risk and treatment response. The urinary stone–associated analytes of 24-hour urine samples from 15 individuals were examined using SLIPS-LAB. To study the metabolic profiles, the data were normalized to typical physiological values of 24-hour urine.
The test results can then be read using a scanner or a cell phone, and the scanned image can then be analyzed using a computer algorithm. All these steps, according to the authors, would take approximately 30 minutes in a physician’s office. An added benefit, they said, is that SLIPS-LAB is more cost-effective than regular, 24-hour testing.
Pak Kin Wong, PhD, professor of biomedical engineering and the principal investigator of the study, said, “We demonstrated that SLIPS-LAB enables the reagent and sample to move themselves and perform the reactions for us. It means the technology doesn’t require a technician to run any test machinery, so it is possible to do the test in non-traditional settings, like a physician’s office or even the patient’s home.”
The authors concluded that SLIPS-LAB will allow detection in a timely and cost-efficient manner and provide actionable diagnostic information and personalized treatment suggestions to individuals with urinary stone disease. The study was published on May 22, 2020 in the journal Science Advances.
Latest Technology News
- Robotic Technology Unveiled for Automated Diagnostic Blood Draws
- ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
- Aptamer Biosensor Technology to Transform Virus Detection
- AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
- AI-Generated Sensors Open New Paths for Early Cancer Detection
- Pioneering Blood Test Detects Lung Cancer Using Infrared Imaging
- AI Predicts Colorectal Cancer Survival Using Clinical and Molecular Features
- Diagnostic Chip Monitors Chemotherapy Effectiveness for Brain Cancer
- Machine Learning Models Diagnose ALS Earlier Through Blood Biomarkers
- Artificial Intelligence Model Could Accelerate Rare Disease Diagnosis
Channels
Molecular Diagnostics
view channel
Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
Glioblastoma is one of the deadliest forms of brain cancer, largely because doctors have no reliable way to determine whether treatments are working in real time. Assessing therapeutic response currently... Read more
Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
Early-stage cancers are notoriously difficult to detect because molecular changes are subtle and often missed by existing screening tools. Many liquid biopsies rely on measuring absolute DNA methylation... Read more
“Lab-On-A-Disc” Device Paves Way for More Automated Liquid Biopsies
Extracellular vesicles (EVs) are tiny particles released by cells into the bloodstream that carry molecular information about a cell’s condition, including whether it is cancerous. However, EVs are highly... Read more
Blood Test Identifies Inflammatory Breast Cancer Patients at Increased Risk of Brain Metastasis
Brain metastasis is a frequent and devastating complication in patients with inflammatory breast cancer, an aggressive subtype with limited treatment options. Despite its high incidence, the biological... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more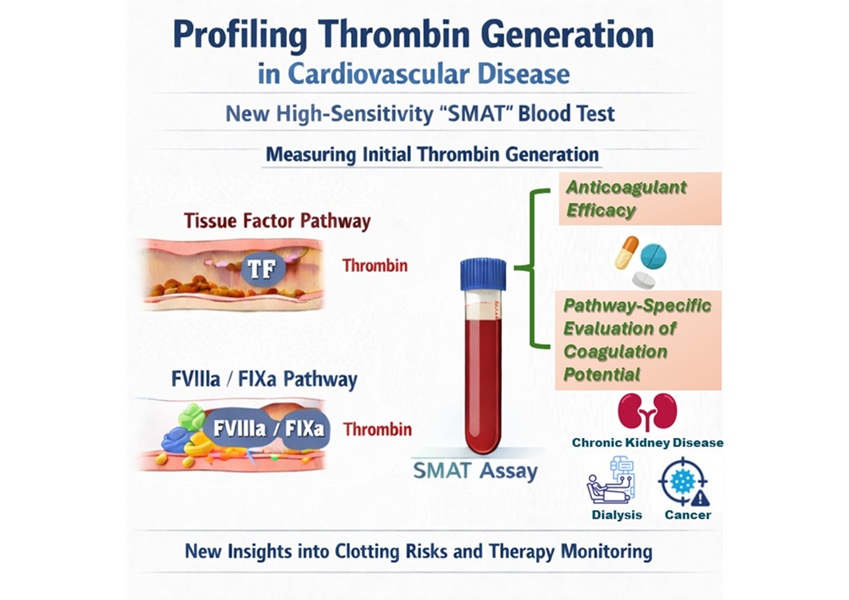
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreAI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
Infections caused by the pathogenic yeast Candida auris pose a significant threat to hospitalized patients, particularly those with weakened immune systems or those who have invasive medical devices.... Read morePathology
view channel
Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Developing new cancer immunotherapies is a slow, costly, and high-risk process, particularly for CAR T cell treatments that must precisely recognize cancer-specific antigens. Small differences in tumor... Read more
First-Of-Its-Kind Test Identifies Autism Risk at Birth
Autism spectrum disorder is treatable, and extensive research shows that early intervention can significantly improve cognitive, social, and behavioral outcomes. Yet in the United States, the average age... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channelNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more
Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
Diasorin (Saluggia, Italy) has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific (Waltham, MA, USA), for the LIAISON NES molecular point-of-care... Read more