POC Diagnostic Tool Introduced for Allergic Disorder Biomarker
|
By LabMedica International staff writers Posted on 20 Sep 2016 |

Image: The Niji test specific disposable cartridge for measuring total immunoglobulin E (IgE) from whole blood (Photo courtesy of Novartis).
Identifying patients with uncontrolled asthma mediated by immunoglobulin E remains an area of high unmet need and it is estimated that in Europe, more than 30 million people are affected by asthma, and approximately half of patients remain uncontrolled.
Current immunoglobulin E tests are performed in central laboratories and it can take up to several weeks before results are known; however point of care testing (P-O-C) in the healthcare provider's office has the potential to reduce the time from consultation to diagnosis and to treatment decision, leading to improved patient outcomes.
Immunoglobulin E (IgE) and the total concentration in blood (Total IgE) is an important biomarker for the diagnosis and management of atopic asthma and other allergic conditions. Elevated IgE can be used as a first indicator for atopic asthma in the general population. In patients with asthma, higher IgE levels are associated with increased asthma severity and reduced lung function.
A novel in-office point of care diagnostic tool, the Niji System and Total IgE Test (Novartis, Basel, Switzerland) has recently been introduced and the test delivers quantitative total IgE levels in about 12 minutes using approximately 30µL of finger stick blood. The test has a dynamic range of 30 to 1,500 IU/mL, allowing for quick in-office diagnosis of IgE-mediated allergic disorders in conjunction with other clinical findings.
The Niji System does not require any sample preparation, lengthy setup, or any calibration procedures. Clinical and non-clinical performance evaluations conducted with the Niji Total IgE Test demonstrated performance comparable to currently marketed reference laboratory tests. The test may be a useful tool for healthcare professionals as an aid in the diagnosis of IgE-mediated allergic disorders in conjunction with other clinical findings in their office setting.
The Niji System is for use by medical professionals and consists of a small desktop analyzer, optional accessories, and test-specific disposable cartridges. It is based on a proprietary pyro-electric technology that supports the design of sensitive, rapid immunoassay tests using unprocessed capillary blood specimens. This allows the development of tests, which quantitate proteins, antibodies, drug molecules and metabolites in capillary blood and other body fluids within 10 to 15 minutes. The system is now cleared for sale within the European Union and in all countries recognizing the CE Mark and the company intends to launch the system in Europe in Q4 2016.
Vas Narasimhan, MD, Global Head of Drug Development and Chief Medical Officer for Novartis, said, “Point of care testing is an important tool for healthcare professionals in order to make informed treatment decisions within a single appointment, thus helping to ensure patients are not lost to follow-up and ultimately improving patient management and outcomes. The Niji System provides a platform for fast and easy blood tests that could potentially be applied across a variety of disease areas.”
Related Links:
Novartis
Current immunoglobulin E tests are performed in central laboratories and it can take up to several weeks before results are known; however point of care testing (P-O-C) in the healthcare provider's office has the potential to reduce the time from consultation to diagnosis and to treatment decision, leading to improved patient outcomes.
Immunoglobulin E (IgE) and the total concentration in blood (Total IgE) is an important biomarker for the diagnosis and management of atopic asthma and other allergic conditions. Elevated IgE can be used as a first indicator for atopic asthma in the general population. In patients with asthma, higher IgE levels are associated with increased asthma severity and reduced lung function.
A novel in-office point of care diagnostic tool, the Niji System and Total IgE Test (Novartis, Basel, Switzerland) has recently been introduced and the test delivers quantitative total IgE levels in about 12 minutes using approximately 30µL of finger stick blood. The test has a dynamic range of 30 to 1,500 IU/mL, allowing for quick in-office diagnosis of IgE-mediated allergic disorders in conjunction with other clinical findings.
The Niji System does not require any sample preparation, lengthy setup, or any calibration procedures. Clinical and non-clinical performance evaluations conducted with the Niji Total IgE Test demonstrated performance comparable to currently marketed reference laboratory tests. The test may be a useful tool for healthcare professionals as an aid in the diagnosis of IgE-mediated allergic disorders in conjunction with other clinical findings in their office setting.
The Niji System is for use by medical professionals and consists of a small desktop analyzer, optional accessories, and test-specific disposable cartridges. It is based on a proprietary pyro-electric technology that supports the design of sensitive, rapid immunoassay tests using unprocessed capillary blood specimens. This allows the development of tests, which quantitate proteins, antibodies, drug molecules and metabolites in capillary blood and other body fluids within 10 to 15 minutes. The system is now cleared for sale within the European Union and in all countries recognizing the CE Mark and the company intends to launch the system in Europe in Q4 2016.
Vas Narasimhan, MD, Global Head of Drug Development and Chief Medical Officer for Novartis, said, “Point of care testing is an important tool for healthcare professionals in order to make informed treatment decisions within a single appointment, thus helping to ensure patients are not lost to follow-up and ultimately improving patient management and outcomes. The Niji System provides a platform for fast and easy blood tests that could potentially be applied across a variety of disease areas.”
Related Links:
Novartis
Latest Technology News
- Robotic Technology Unveiled for Automated Diagnostic Blood Draws
- ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
- Aptamer Biosensor Technology to Transform Virus Detection
- AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
- AI-Generated Sensors Open New Paths for Early Cancer Detection
- Pioneering Blood Test Detects Lung Cancer Using Infrared Imaging
- AI Predicts Colorectal Cancer Survival Using Clinical and Molecular Features
- Diagnostic Chip Monitors Chemotherapy Effectiveness for Brain Cancer
- Machine Learning Models Diagnose ALS Earlier Through Blood Biomarkers
- Artificial Intelligence Model Could Accelerate Rare Disease Diagnosis
Channels
Clinical Chemistry
view channel
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read more
Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
Chronic kidney disease (CKD) affects more than 1 in 7 Americans and is strongly associated with cardiovascular complications, which account for more than half of deaths among people with CKD.... Read moreMolecular Diagnostics
view channel
Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
Glioblastoma is one of the deadliest forms of brain cancer, largely because doctors have no reliable way to determine whether treatments are working in real time. Assessing therapeutic response currently... Read more
Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
Early-stage cancers are notoriously difficult to detect because molecular changes are subtle and often missed by existing screening tools. Many liquid biopsies rely on measuring absolute DNA methylation... Read more
“Lab-On-A-Disc” Device Paves Way for More Automated Liquid Biopsies
Extracellular vesicles (EVs) are tiny particles released by cells into the bloodstream that carry molecular information about a cell’s condition, including whether it is cancerous. However, EVs are highly... Read more
Blood Test Identifies Inflammatory Breast Cancer Patients at Increased Risk of Brain Metastasis
Brain metastasis is a frequent and devastating complication in patients with inflammatory breast cancer, an aggressive subtype with limited treatment options. Despite its high incidence, the biological... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more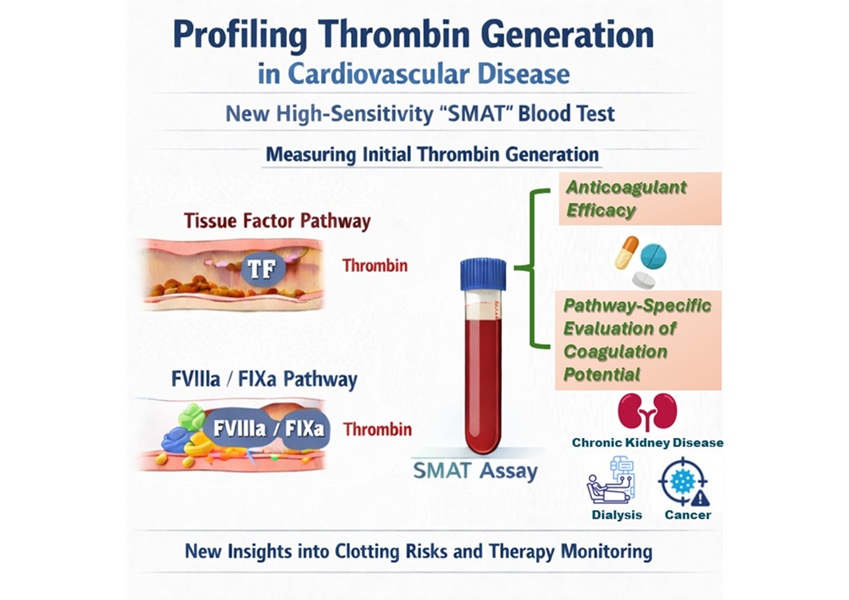
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreMicrobiology
view channel
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreAI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
Infections caused by the pathogenic yeast Candida auris pose a significant threat to hospitalized patients, particularly those with weakened immune systems or those who have invasive medical devices.... Read morePathology
view channel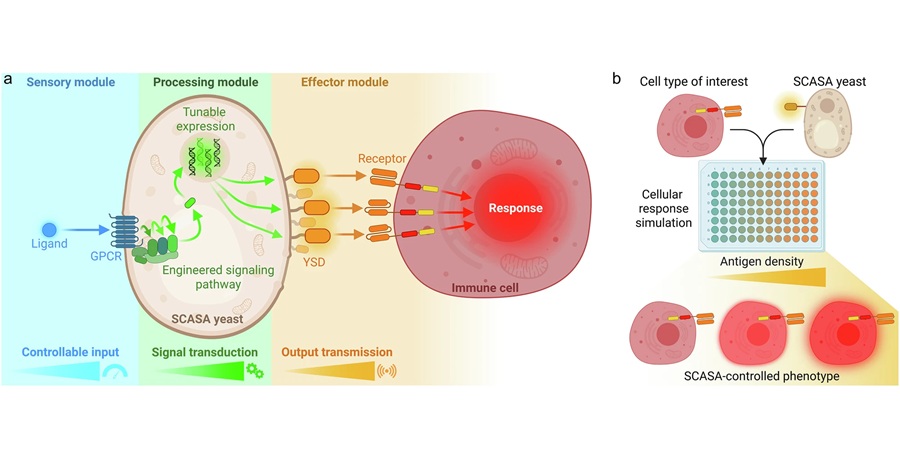
Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Developing new cancer immunotherapies is a slow, costly, and high-risk process, particularly for CAR T cell treatments that must precisely recognize cancer-specific antigens. Small differences in tumor... Read more
First-Of-Its-Kind Test Identifies Autism Risk at Birth
Autism spectrum disorder is treatable, and extensive research shows that early intervention can significantly improve cognitive, social, and behavioral outcomes. Yet in the United States, the average age... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channelNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more
Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
Diasorin (Saluggia, Italy) has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific (Waltham, MA, USA), for the LIAISON NES molecular point-of-care... Read more