One-Tube RNA Ligation and Amplification Method for Rapid Detection of Drug Resistant HIV
|
By LabMedica International staff writers Posted on 28 Oct 2015 |
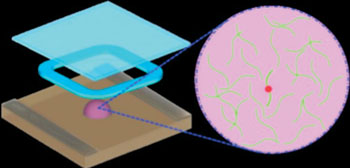
Image: A schematic diagram of a new method that rapidly analyzes the RNA (green strands) of HIV for mutations (red dot) that convey drug resistance. The system does not require transcription of RNA to DNA, as current technologies do, and works within one solution (purple droplet) (Photo courtesy of Dr. Lei Zhang, Brown University).
By not requiring transcription of RNA to DNA, a novel one-tube method allows the rapid detection of drug resistant strains of HIV (human immunodeficiency virus).
In order to detect point mutations in RNA retroviruses, conventional ligase-mediated approaches require the reverse transcription of viral RNA genomes into DNA before separate ligation and amplification steps can be carried out.
To simplify this process, investigators at Brown University (Providence, RI, USA) developed one-step ligation on RNA amplification (LRA) method for the direct detection of RNA point mutations. The system operates directly on viral RNA rather than requiring extra, potentially error-prone steps to examine DNA derived from RNA. In a single tube, the system first combines two engineered probes (ligation). If a mutation is present, it then makes many copies of those combined probes (amplification) for detection.
The investigators used this technique for the detection of a common, clinically relevant HIV-1 reverse transcriptase drug-resistant point mutation, K103N, and compared it with allele-specific PCR and pyrosequencing methodology.
They reported in the November 2015 issue of the Journal of Molecular Diagnostics that the LRA test was sensitive enough to detect the K103N mutation in concentrations as low as one mutant per 10,000 strands of normal viral RNA. The LRA test required about two hours while the alternative technologies took as long as eight hours.
"LRA (ligation on RNA amplification) uniquely optimizes two enzymatic reactions—RNA-based ligation, and quantitative PCR (polymerase chain reaction) amplification—into a single system," said senior author Dr. Anubhav Tripathi, professor of engineering at Brown University. "Each HIV contains about 10,000 nucleotides, or building blocks, in its genetic material, and a drop of blood from a patient with resistant HIV can contain thousands to millions of copies of HIV. To find that one virus, out of thousands to millions, which is mutated at just a single nucleotide is like finding a needle in a haystack."
So far the LRA test has been shown to work on RNA that was derived from laboratory HIV strains, but it has not yet been applied to samples from circulating viruses from AIDS patients.
Related Links:
Brown University
In order to detect point mutations in RNA retroviruses, conventional ligase-mediated approaches require the reverse transcription of viral RNA genomes into DNA before separate ligation and amplification steps can be carried out.
To simplify this process, investigators at Brown University (Providence, RI, USA) developed one-step ligation on RNA amplification (LRA) method for the direct detection of RNA point mutations. The system operates directly on viral RNA rather than requiring extra, potentially error-prone steps to examine DNA derived from RNA. In a single tube, the system first combines two engineered probes (ligation). If a mutation is present, it then makes many copies of those combined probes (amplification) for detection.
The investigators used this technique for the detection of a common, clinically relevant HIV-1 reverse transcriptase drug-resistant point mutation, K103N, and compared it with allele-specific PCR and pyrosequencing methodology.
They reported in the November 2015 issue of the Journal of Molecular Diagnostics that the LRA test was sensitive enough to detect the K103N mutation in concentrations as low as one mutant per 10,000 strands of normal viral RNA. The LRA test required about two hours while the alternative technologies took as long as eight hours.
"LRA (ligation on RNA amplification) uniquely optimizes two enzymatic reactions—RNA-based ligation, and quantitative PCR (polymerase chain reaction) amplification—into a single system," said senior author Dr. Anubhav Tripathi, professor of engineering at Brown University. "Each HIV contains about 10,000 nucleotides, or building blocks, in its genetic material, and a drop of blood from a patient with resistant HIV can contain thousands to millions of copies of HIV. To find that one virus, out of thousands to millions, which is mutated at just a single nucleotide is like finding a needle in a haystack."
So far the LRA test has been shown to work on RNA that was derived from laboratory HIV strains, but it has not yet been applied to samples from circulating viruses from AIDS patients.
Related Links:
Brown University
Latest BioResearch News
- Barcoded DNA Sheds Light on Hidden Complexities in Breast Cancer Detection
- CRISPR-Based Platform Pinpoints Drivers of Acute Myeloid Leukemia in Patient Cells
- Protective Brain Protein Emerges as Biomarker Target in Alzheimer’s Disease
- Genome Analysis Predicts Likelihood of Neurodisability in Oxygen-Deprived Newborns
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- Gene Fusion Protein Proposed as Prostate Cancer Biomarker
- NIV Test to Diagnose and Monitor Vascular Complications in Diabetes
- Semen Exosome MicroRNA Proves Biomarker for Prostate Cancer
- Genetic Loci Link Plasma Lipid Levels to CVD Risk
- Newly Identified Gene Network Aids in Early Diagnosis of Autism Spectrum Disorder
- Link Confirmed between Living in Poverty and Developing Diseases
- Genomic Study Identifies Kidney Disease Loci in Type I Diabetes Patients
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Simple One-Hour Saliva Test Detects Common Cancers
Early detection is critical for improving cancer outcomes, yet many diagnostic tests rely on invasive procedures such as blood draws or biopsies. Researchers are exploring simpler approaches that could... Read more
Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
Chemotherapy is often highly effective for germ cell tumors, but in a subset of patients, the disease does not respond well to standard treatment. For these individuals, doctors may consider high-dose... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read more
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read morePathology
view channel
World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
Blood sampling is one of the most common clinical procedures, but it can be difficult or uncomfortable for many patients, especially older adults or individuals with certain medical conditions.... Read more
Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
Polymerase Chain Reaction (PCR) testing became widely recognized during the COVID-19 pandemic as a powerful method for detecting viruses such as SARS-CoV-2. PCR belongs to a group of diagnostic methods... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more






















