Certain MicroRNAs Stimulate Regeneration of Adult Heart Tissue
|
By LabMedica International staff writers Posted on 31 Mar 2015 |
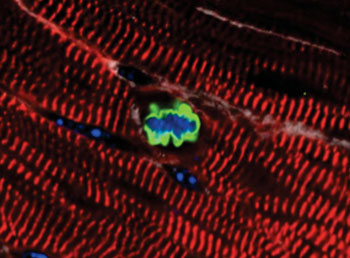
Image: An adult cardiomyocyte has re-entered the cell cycle after expression of miR302-367 (Photo courtesy of the laboratory of Dr. Edward Morrisey, University of Pennsylvania).
Cardiac disease researchers working with a mouse model have discovered that by inducing a subset of microRNAs (miRNAs) that are active during development but silenced in the adult they could cause damaged adult heart tissue to regenerate.
The mammalian heart has limited capacity to regenerate after injury in part due to ineffective reactivation of cardiomyocyte proliferation. Investigators at the University of Pennsylvania (Philadelphia, USA) recently found that the microRNA cluster miR302-367 was important for cardiomyocyte proliferation during development and was sufficient to induce cardiomyocyte proliferation in the adult and promote cardiac regeneration. MiRNAs are fragments of RNA about 20 nucleotides long that block gene expression by attaching to molecules of messenger RNA (mRNA) in a fashion that prevents them from transmitting the protein synthesizing instructions they had received from the DNA.
The investigators reported in the March 18, 2015, online edition of the journal Science Translational Medicine that in their mouse model loss of miR302-367 led to decreased cardiomyocyte proliferation during development. In contrast, elevated miR302-367 expression led to a profound increase in cardiomyocyte proliferation, in part through repression of the Hippo signal transduction pathway. The Hippo signaling pathway controls organ size in animals through the regulation of cell proliferation and apoptosis. The pathway takes its name from one of its key signaling components, the protein kinase Hippo (Hpo). Mutations in this gene lead to tissue overgrowth, or a "hippopotamus"-like phenotype.
Induced expression of miR302-367 in adult animals reactivated the cell cycle in cardiomyocytes, resulting in reduced scar formation after experimental myocardial infarction. Furthermore, the number of heart muscle cells in these mice was found to increase. However, long-term expression of miR302-367 induced cardiomyocyte dedifferentiation and dysfunction, suggesting that persistent reactivation of the cell cycle in postnatal cardiomyocytes was not desirable. This limitation was overcome by transient systemic application of synthetic microRNAs that mimicked miR302-367, leading to increased cardiomyocyte proliferation and mass, decreased fibrosis, and improved function after injury.
"The Hippo pathway normally represses cell proliferation when it is turned on. The cluster miR302-367 targets three of the major kinase components in the Hippo pathway, reducing pathway activity, which allows cardiomyocytes to re-enter the cell cycle and begin to regrow heart muscle," said senior author Dr. Edward E. Morrisey, professor of medicine and cell and developmental biology at the University of Pennsylvania. "This is a case of repressing a repressor."
"Persistent reactivation of the cell cycle in adult cardiomyocytes could be harmful and causes the heart to fail," said Dr. Morrisey. "We overcame this limitation by injecting synthetic microRNAs with a short half-life called mimics into the mice. The next stage in this study is to determine whether miRNA mimics will work in a larger animal model and to collaborate with bioengineers to create a local delivery system for the heart, rather than giving it systemically."
Related Links:
University of Pennsylvania
The mammalian heart has limited capacity to regenerate after injury in part due to ineffective reactivation of cardiomyocyte proliferation. Investigators at the University of Pennsylvania (Philadelphia, USA) recently found that the microRNA cluster miR302-367 was important for cardiomyocyte proliferation during development and was sufficient to induce cardiomyocyte proliferation in the adult and promote cardiac regeneration. MiRNAs are fragments of RNA about 20 nucleotides long that block gene expression by attaching to molecules of messenger RNA (mRNA) in a fashion that prevents them from transmitting the protein synthesizing instructions they had received from the DNA.
The investigators reported in the March 18, 2015, online edition of the journal Science Translational Medicine that in their mouse model loss of miR302-367 led to decreased cardiomyocyte proliferation during development. In contrast, elevated miR302-367 expression led to a profound increase in cardiomyocyte proliferation, in part through repression of the Hippo signal transduction pathway. The Hippo signaling pathway controls organ size in animals through the regulation of cell proliferation and apoptosis. The pathway takes its name from one of its key signaling components, the protein kinase Hippo (Hpo). Mutations in this gene lead to tissue overgrowth, or a "hippopotamus"-like phenotype.
Induced expression of miR302-367 in adult animals reactivated the cell cycle in cardiomyocytes, resulting in reduced scar formation after experimental myocardial infarction. Furthermore, the number of heart muscle cells in these mice was found to increase. However, long-term expression of miR302-367 induced cardiomyocyte dedifferentiation and dysfunction, suggesting that persistent reactivation of the cell cycle in postnatal cardiomyocytes was not desirable. This limitation was overcome by transient systemic application of synthetic microRNAs that mimicked miR302-367, leading to increased cardiomyocyte proliferation and mass, decreased fibrosis, and improved function after injury.
"The Hippo pathway normally represses cell proliferation when it is turned on. The cluster miR302-367 targets three of the major kinase components in the Hippo pathway, reducing pathway activity, which allows cardiomyocytes to re-enter the cell cycle and begin to regrow heart muscle," said senior author Dr. Edward E. Morrisey, professor of medicine and cell and developmental biology at the University of Pennsylvania. "This is a case of repressing a repressor."
"Persistent reactivation of the cell cycle in adult cardiomyocytes could be harmful and causes the heart to fail," said Dr. Morrisey. "We overcame this limitation by injecting synthetic microRNAs with a short half-life called mimics into the mice. The next stage in this study is to determine whether miRNA mimics will work in a larger animal model and to collaborate with bioengineers to create a local delivery system for the heart, rather than giving it systemically."
Related Links:
University of Pennsylvania
Latest BioResearch News
- CRISPR-Based Platform Pinpoints Drivers of Acute Myeloid Leukemia in Patient Cells
- Protective Brain Protein Emerges as Biomarker Target in Alzheimer’s Disease
- Genome Analysis Predicts Likelihood of Neurodisability in Oxygen-Deprived Newborns
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- Gene Fusion Protein Proposed as Prostate Cancer Biomarker
- NIV Test to Diagnose and Monitor Vascular Complications in Diabetes
- Semen Exosome MicroRNA Proves Biomarker for Prostate Cancer
- Genetic Loci Link Plasma Lipid Levels to CVD Risk
- Newly Identified Gene Network Aids in Early Diagnosis of Autism Spectrum Disorder
- Link Confirmed between Living in Poverty and Developing Diseases
- Genomic Study Identifies Kidney Disease Loci in Type I Diabetes Patients
- Liquid Biopsy More Effective for Analyzing Tumor Drug Resistance Mutations
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Swab Test Helps Transplant Patients Receive Right Anti-Rejection Medication Dose
Tacrolimus is widely used to prevent organ rejection in transplant recipients, but achieving the correct dose early is critical. If levels are too low, the transplanted organ may be rejected; if too high,... Read more
Blood Test Predicts Which Bladder Cancer Patients May Safely Skip Surgery
Muscle-invasive bladder cancer (MIBC) often requires removal of the bladder, a procedure that significantly affects quality of life and carries a risk of complications. While newer treatments have improved... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read more
Hidden Gut Viruses Linked to Colorectal Cancer Risk
Colorectal cancer (CRC) remains a leading cause of cancer mortality in many Western countries, and existing risk-stratification approaches leave substantial room for improvement. Although age, diet, and... Read morePathology
view channel
Molecular Imaging to Reduce Need for Melanoma Biopsies
Melanoma is the deadliest form of skin cancer and accounts for the vast majority of skin cancer-related deaths. Because early melanomas can closely resemble benign moles, clinicians often rely on visual... Read more
Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
Urine testing is a critical, non-invasive diagnostic tool used to detect conditions such as pregnancy, urinary tract infections, metabolic disorders, cancer, and kidney disease. However, contaminated or... Read moreTechnology
view channel
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read more
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more






















