Seegene Showcases All-in-One Platform for Molecular Diagnostic Testing for Infectious Diseases
|
By LabMedica International staff writers Posted on 25 Apr 2022 |

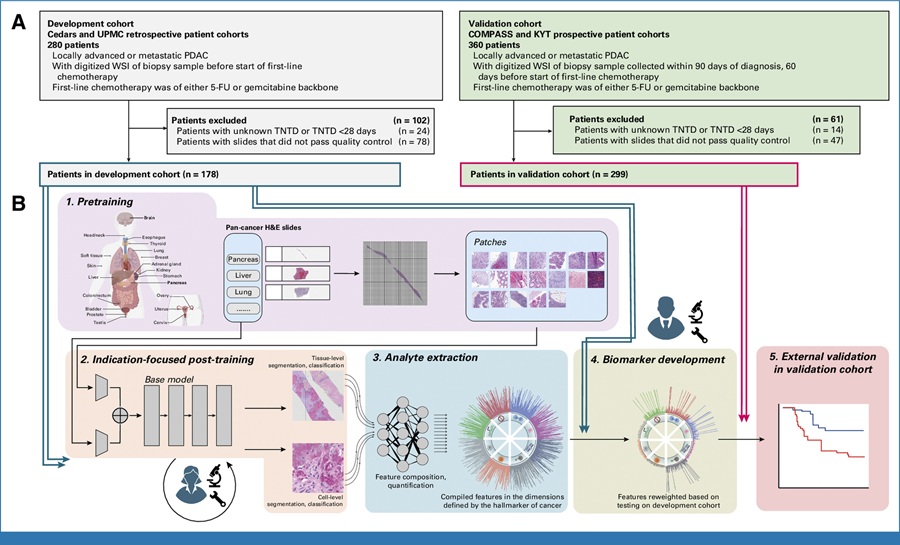
Seegene, Inc. (Seoul, Korea) showcased its first fully automated MDx system, STARlet-AIOS: All-in-One System at the 32nd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID 2022).
ECCMID is the world’s premier clinical microbiology & infectious diseases event that brings together experts from many fields to present their latest findings, guidelines and experiences to an audience of over 14,000 colleagues. This year, the congress featured a hybrid format to facilitate an onsite and online experience for attendees allowing for remote and face-to-face opportunities to take part in the multifaceted program. During this event, Seegene showcased AIOS – its first fully automated MDx system. AIOS provides high-throughput real-time PCR workflow starting from nucleic acid extraction, then, real-time PCR, and finally result interpretation. AIOS is a fully automated syndromic MDx system designed in a modular concept while it compiles Seegene’s cutting-edge high multiplex real-time PCR technologies.
A noteworthy feature of AIOS is that its MDx system is composed of independent and detachable modules together with its proprietary SW architecture. Unlike others’ single-body system, AIOS is designed with an extraction/liquid handler and PCR instrument being integrated with an in-house developed robotic arm module. Hospitals and laboratories can either purchase the full package of ‘AIOS’ system or integrate their existing instruments on-site if they already have Seegene’s liquid handler and real-time PCR instrument.
Some of its other highlights include: ‘high-throughput automated syndromic MDx system’. It also adopts a variety of Seegene’s assays that are currently available on the market, which allows for symptom-based tests. And finally, it can identify the exact cause of a specific symptom with a single tube, which will enable both cost- and time- effective tests. The AIOS is ideal for small-to-medium sized hospitals due to its unique modular and assay features.
Related Links:
Seegene, Inc.
Latest ECCMID 2022 News
- BD Presents Latest Automated Solutions to Combat Antimicrobial Resistance at ECCMID 2022
- Boditech Presents Latest IVD Solutions at ECCMID 2022
- CerTest Presents VIASURE Complete Solution for Improved Molecular Diagnostic Workflow
- BioVendor Presents Its Portfolio of IVD Products and Technologies for Lab Automation
- Bio-Rad Exhibits Complete Infectious Disease Testing Portfolio at ECCMID 2022
- Siemens Presents Its Full Portfolio of COVID-19 Testing Solutions at ECCMID 2022
- MeMed Highlights Pioneering Blood Test for Accurately Distinguishing Between Bacterial and Viral Infection
- Abbott Highlights Rapid Diagnostic Platforms to Complement Centralized RT-PCR Testing at ECCMID 2022
- Beckman Coulter Presents Virtual Showcase of MicroScan Microbiology Solutions
- Randox Presents Vivalytic Universal, Fully Automated All-in-One Solution for Molecular Diagnostics at ECCMID 2022
- Meridian Bioscience Exhibits Curian Immunofluorescent Testing Platform at ECCMID 2022
- Copan Showcases Fully Automated Solutions for Antibiotic Susceptibility Testing at ECCMID 2022
- Autobio Diagnostics Showcases Automated Microbial Identification and ID-AST Solutions at ECCMID 2022
- Altona Diagnostics Exhibits AltoStar Product Range at ECCMID 2022
- Alifax Presents First Automated System for Bacterial Culture and Susceptibility Testing at ECCMID 2022
- LumiraDx Demonstrates Use of LumiraDx Platform in Infectious Disease Testing at ECCMID 2022
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreMolecular Diagnostics
view channel
AI-Powered Blood Test Detects Early Pancreatic Cancer with More Than 90% Accuracy
Pancreatic cancer is one of the most lethal cancers, often referred to as the “King of Cancers” because symptoms usually appear only at advanced stages. As a result, most patients are diagnosed late, and... Read more
AI-Powered Blood Test Flags Relapse Risk Earlier After Transplant
Relapse after allogeneic hematopoietic cell transplant is a major cause of mortality in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), and standard monitoring can miss early warning signals.... Read more
World’s First Portable POC Test Simultaneously Detects Four Common STIs in One Hour
Sexually transmitted infections (STIs) often present with similar symptoms, making accurate diagnosis challenging without laboratory testing. Delays in identifying the exact infection can lead to inappropriate... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read more
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Agilent Technologies Acquires Pathology Diagnostics Company Biocare Medical
Agilent Technologies (Santa Clara, CA, USA) has entered into a definitive agreement to acquire Biocare Medical (Pacheco, CA, USA), expanding its pathology portfolio through the addition of highly complementary... Read more
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more