Siemens Presents Its Full Portfolio of COVID-19 Testing Solutions at ECCMID 2022
Posted on 26 Apr 2022
Siemens Healthineers (Erlangen, Germany) presented its full portfolio of COVID-19 testing solutions, from a highly sensitive RT-PCR test that can detect Omicron sublineage BA.21 to high throughput or point of care antigen and antibody assays, at ECCMID 2022.
Changing COVID-19 testing demands require clinical laboratories to keep up with an increasing number of patients, samples, and emerging variants. Siemens provides the flexibility laboratories want for the clinical results needed with its COVID-19 testing solutions. The company’s one-hour integrated symposium at the event focused on testing for respiratory viruses, including SARS-CoV-2 and other seasonal respiratory viruses, using molecular methods. Siemens highlighted the challenging and dynamic nature of this field along with a discussion on how labs are addressing the evolution of testing needs, including SARS-CoV-2 variants, seasonal viruses, and potential future outbreaks.
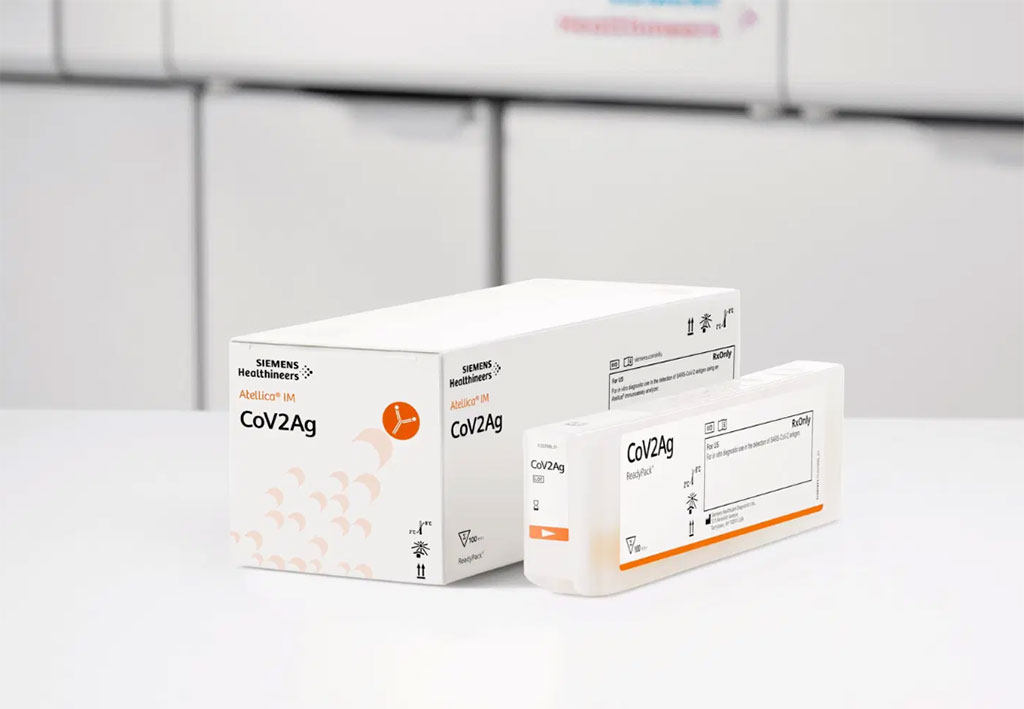
Visitors at the Siemens ECCMID booth learnt how its testing solutions can help laboratories deliver more impactful results for the detection and management of COVID-19. In addition, Siemens offered an interactive platform with recorded presentations and whitepapers highlighting its COVID-19 testing solutions, including reflex testing for emerging variants. Under molecular testing which is considered the gold standard for accurate and early detection of COVID-19 infection in patients, Siemens demonstrated its FTD SARS-CoV-2 Assay, which is intended for the initial diagnosis of the infection of the SARS-CoV-2 virus. The kit is comprised of a single-well dual target assay for the specific detection of SARS-CoV-2 and has also been shown to detect emergent SARS-CoV-2 variants.
High-throughput, central lab-based SARS-CoV-2 antigen testing is a cost-effective and highly scalable complement to molecular RT-PCR diagnostic testing in the fight against COVID-19. For large-scale testing and diagnosis, Siemens demonstrated its high-throughput SARS-CoV-2 Antigen Assay that offers lab-based antigen testing for rapid virus detection with time to first result in as little as 26 minutes. The SARS-CoV-2 Antigen Assays are available on Siemens Atellica IM Analyzer and ADVIA Centaur XP/XPT Systems.
Rapid and accurate antibody testing on a large scale is vital to address the challenges of the COVID-19 pandemic. Over time, SARS-CoV-2 IgG antibodies remain the primary antibodies present. Siemens highlighted its SARS-CoV-2 IgG (sCOVG) assay and the SARS-CoV-2 Total assay for the qualitative and quantitative detection of neutralizing antibodies to the SARS-CoV-2 virus. The SARS-CoV-2 Total (COV2T) Assay can be used effectively for broad population testing and produces results in as little as 10 minutes on the Atellica IM Analyzer with a capacity to process up to 440 assays per hour. On the other hand, Siemens Atellica IM and ADVIA Centaur SARS-CoV-2 IgG (sCOVG) assays measure neutralizing IgG antibodies to SARS-CoV-2 in the blood to help clinicians assess the level of an individual's immune response over time.
Related Links:
Siemens Healthineers