AACC: Better Quality and Patient-Friendliness Needed in Direct Testing
|
By LabMedica International staff writers Posted on 27 Jul 2015 |
In a new position statement on direct-to-consumer (DTC) laboratory testing, the American Association for Clinical Chemistry (AACC; Washington DC, USA) emphasizes the need for patient-friendly reports and for sufficient transparency about quality of tests and results.
DTC testing allows people to order medical tests directly from a lab, without going through their healthcare provider. Noting the paradigm shift among consumers seeking greater control over their own healthcare, AACC has issued a position statement that emphasizes DTC test results must be accurate and easily understood—an area where laboratory medicine professionals play a vital role.
State laws have limited to physicians the ordering of lab tests, but as people have become more engaged in managing their own healthcare, this model has begun to change. Currently, 37 states and the District of Columbia permit consumers to order some or all of their laboratory tests without involvement of a physician. Individuals can also buy over-the-counter test kits or get laboratory services from non-traditional settings such as retail centers. These DTC lab tests can provide invaluable information to individuals about their health status in a timely and convenient manner. However, many healthcare providers and policymakers are concerned that some of these tests may be of questionable quality and value, or that consumers might not have enough background knowledge to make sound decisions based on their test results.
To enhance patient benefit, AACC urges the Centers for Medicare and Medicaid Services and the US Food and Drug Administration (FDA) to require that DTC testing providers disclose sufficient information about their products and services, enabling consumers to make well-informed decisions. These providers should provide: user-friendly descriptions of risks, benefits, and limitations of all tests offered; clear and understandable reports of test results, with enough information to assist in decision-making; prominent instructions to contact a qualified healthcare provider with any questions or concerns; and a comprehensive, public listing of tests offered and prices charged.
Laboratory medicine professionals are integral to this consumer-driven process. AACC encourages lab professionals to collaborate with federal agencies to inform the public about the costs, benefits, interpretation, and limitations of DTC tests. Likewise, consumers should consult qualified professionals in making decisions about their healthcare.
“DTC laboratory testing is a key element of ongoing efforts to empower people in decisions affecting their healthcare,” said AACC CEO Janet B. Kreizman, “AACC supports expanding consumer access to high-quality DTC testing services, and urges policymakers to ensure that these services have demonstrated clinical validity and utility and make a positive impact on patient outcomes.”
Related Links:
American Association for Clinical Chemistry (AACC)
AACC position statement on direct-to-consumer testing
DTC testing allows people to order medical tests directly from a lab, without going through their healthcare provider. Noting the paradigm shift among consumers seeking greater control over their own healthcare, AACC has issued a position statement that emphasizes DTC test results must be accurate and easily understood—an area where laboratory medicine professionals play a vital role.
State laws have limited to physicians the ordering of lab tests, but as people have become more engaged in managing their own healthcare, this model has begun to change. Currently, 37 states and the District of Columbia permit consumers to order some or all of their laboratory tests without involvement of a physician. Individuals can also buy over-the-counter test kits or get laboratory services from non-traditional settings such as retail centers. These DTC lab tests can provide invaluable information to individuals about their health status in a timely and convenient manner. However, many healthcare providers and policymakers are concerned that some of these tests may be of questionable quality and value, or that consumers might not have enough background knowledge to make sound decisions based on their test results.
To enhance patient benefit, AACC urges the Centers for Medicare and Medicaid Services and the US Food and Drug Administration (FDA) to require that DTC testing providers disclose sufficient information about their products and services, enabling consumers to make well-informed decisions. These providers should provide: user-friendly descriptions of risks, benefits, and limitations of all tests offered; clear and understandable reports of test results, with enough information to assist in decision-making; prominent instructions to contact a qualified healthcare provider with any questions or concerns; and a comprehensive, public listing of tests offered and prices charged.
Laboratory medicine professionals are integral to this consumer-driven process. AACC encourages lab professionals to collaborate with federal agencies to inform the public about the costs, benefits, interpretation, and limitations of DTC tests. Likewise, consumers should consult qualified professionals in making decisions about their healthcare.
“DTC laboratory testing is a key element of ongoing efforts to empower people in decisions affecting their healthcare,” said AACC CEO Janet B. Kreizman, “AACC supports expanding consumer access to high-quality DTC testing services, and urges policymakers to ensure that these services have demonstrated clinical validity and utility and make a positive impact on patient outcomes.”
Related Links:
American Association for Clinical Chemistry (AACC)
AACC position statement on direct-to-consumer testing
Latest AACC 2015 News
- Automated Molecular Diagnostics System Presented at AACC 2015
- Portable Molecular Diagnostics System Unveiled At 2015 AACC
- Expanded Steroid Control Launched at the 2015 AACC Annual Meeting
- Innovative New Technology to Provide Plastic-Exterior Components with Glass Interior, Presented at AACC 2015
- Eco-Friendly Immunoassay Reagents Featured at AACC 2015
- Low Cost Point-of-Care DNA Amplification Test for Chlamydia Infection Demonstrated at the 2015 AACC Annual Meeting
- Inexpensive Multipurpose Point-of-Care Analyzer Unveiled at 2015 AACC Annual Meeting
- State-of-the-Art Automated Laboratory Systems Highlighted at the 2015 AACC Annual Meeting
- Siemens Showcases Multiple New IVD Solutions at AACC 2015
- New HPLC Quadruples Clinical Throughput Capabilities, Displayed at AACC 2015
- Diagnostic Test that Measures Active Renin in Hypertension Displayed at the 2015 AACC Annual Meeting
- Hair Testing May Offer Insights into Asthma-Related Complications in Pregnancy
- Two Newly Developed Tests May Better Diabetes Diagnosis and Monitoring
- CE Marking of Theranostic Monitoring Test Announced at 2015 AACC Annual Meeting
- Ebola Rapid Lateral Flow Test Previewed at the 2015 AACC Annual Meeting
- Clinical Chemistry Instruments and Reagents Under Scrutiny at the 2015 AACC Annual Meeting
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Simple One-Hour Saliva Test Detects Common Cancers
Early detection is critical for improving cancer outcomes, yet many diagnostic tests rely on invasive procedures such as blood draws or biopsies. Researchers are exploring simpler approaches that could... Read more
Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
Chemotherapy is often highly effective for germ cell tumors, but in a subset of patients, the disease does not respond well to standard treatment. For these individuals, doctors may consider high-dose... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read more
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read morePathology
view channel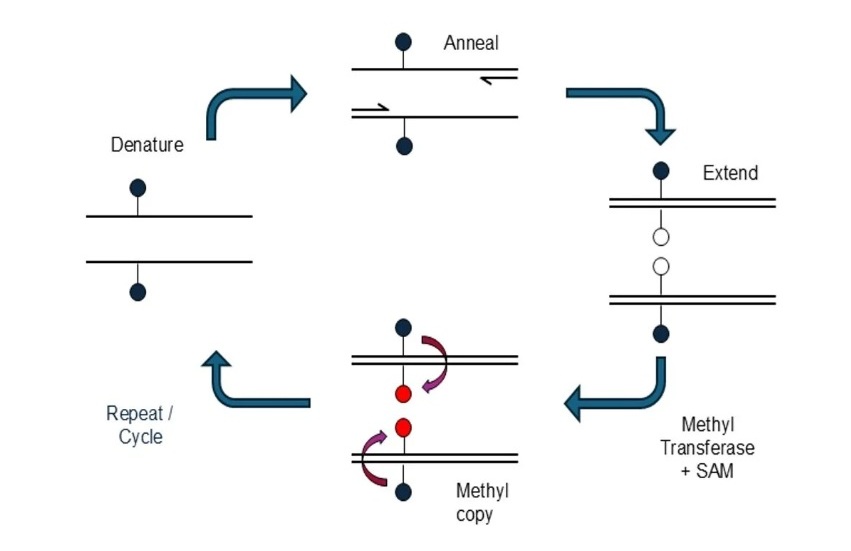
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more























