MCED Could Be Valuable Supplement to Traditional Cancer Screening Approaches
Posted on 10 Jul 2025
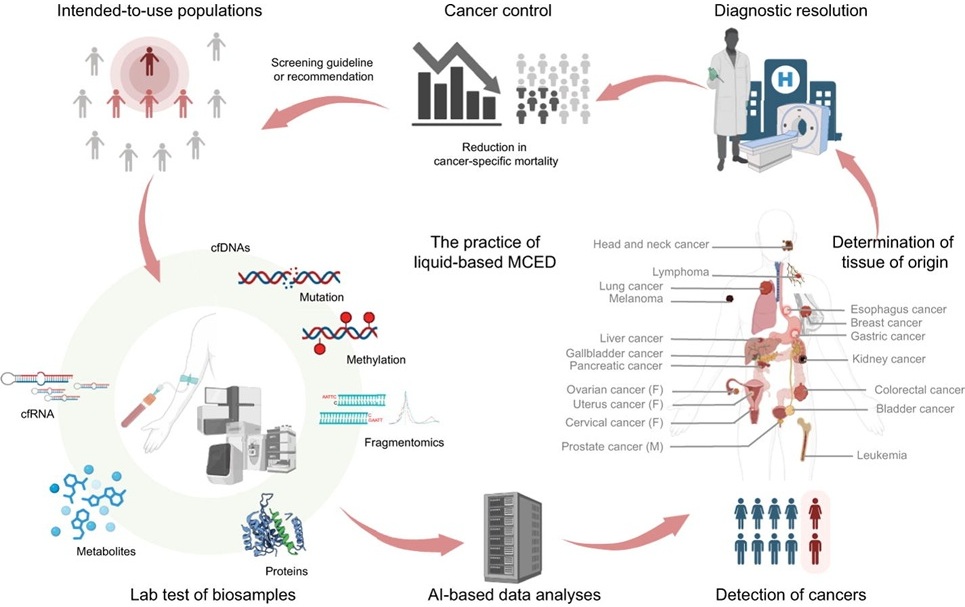
Current cancer screening methods are limited in scope, primarily focusing on a few high-incidence cancer types, such as those detected by mammography, colonoscopy, and low-dose computed tomography (LDCT). These traditional methods often have high false-positive rates and require invasive procedures, leading to poor patient adherence and limited overall effectiveness. Additionally, detecting cancer early—when it is most treatable—remains a challenge. Liquid biopsy-based multi-cancer early detection (MCED) has been identified as a potential breakthrough, offering a non-invasive, multi-cancer diagnostic approach. By analyzing biological markers such as cfDNA, cfRNA, and proteins from blood or body fluids, MCED can detect multiple cancers simultaneously and potentially localize the tissue of origin, offering a promising solution for earlier and more effective cancer detection.
Researchers from the Chinese Academy of Medical Sciences (CACMS, Beijing, China) and collaborators have explored the road for liquid biopsy-based MCED from evidence to implementation in a paper published in Science Bulletin. The technology utilizes liquid biopsy to analyze biomarkers in blood or other body fluids, leveraging AI algorithms to enhance detection accuracy. This approach offers broad population and cancer-type coverage, minimal invasiveness, and high patient compliance. The MCED method works by analyzing cfDNA, cfRNA, and proteins to detect cancer markers, expanding screening to a wider range of cancers. While MCED holds potential, further validation through clinical trials and real-world evidence is needed to establish its clinical efficacy. The MCED method has been evaluated through various clinical trials, and results show significant promise.
The findings indicate that MCED technology offers improved sensitivity and specificity over existing screening techniques, enabling the detection of cancers earlier and with greater accuracy. The technology could also potentially reduce late-stage cancer incidence, leading to better outcomes and lower healthcare costs. However, challenges remain, such as balancing surrogate endpoints with actual public health benefits, improving early detection for cancers with low cfDNA shedding, and minimizing false positives and mislocalization errors. Researchers are working to refine the technology, focusing on clinical validation, cost control, and enhancing evidence generation to support MCED’s future implementation as a complementary tool to existing cancer screening approaches.














