Recombinant Antigen-Based ELISA Evaluated for Syphilis
|
By LabMedica International staff writers Posted on 28 Aug 2013 |
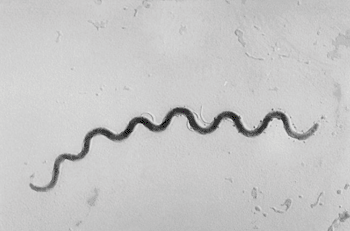
Image: Photomicrograph of Treponema pallidum (Photo courtesy of Susan Lindsley).
The diagnostic performance of the latest screening enzyme-linked immunosorbent assay (ELISA) for syphilis has been compared with the currently used treponemal tests.
The etiological agent of syphilis, Treponema pallidum, cannot be cultured and there is no single optimal alternative test. Serological testing is the most frequently used approach in laboratory diagnosis of the disease.
Scientists at Sekisui Virotech (Rüsselsheim, Germany) compared their Treponema pallidum Screen ELISA with standard tests. These tests included the fluorescent treponemal antibody absorption (FTA-ABS, Zeus Scientific; Branchburg, NJ, USA) test, which is an indirect fluorescent antibody technique; the T. pallidum particle agglutination (TPPA, Fujirebio; Hoofddorp, the Netherlands) test, which is a qualitative assay for the detection of antibodies to T. pallidum in serum or plasma. The most relevant test used for comparison was the Trep-Sure ELISA (Phoenix Bio-Tech Corporation; Mississauga, ON, Canada).
To establish the sensitivity and specificity of the Virotech Screen, 421 serum samples from different panels of infected and non-infected patients, sera from seronegative pregnant women as well as international syphilis standard sera and panels were tested. In comparison to combined TPPA/FTA-abs tests, Phoenix Trep-Sure and Virotech Screen demonstrated a sensitivity of 100% and a specificity of 93.9% and 98.3%, respectively.
All samples of a well-defined syphilis serum panel were correctly identified by the Virotech test, whereas the Phoenix test identified two Treponema negative samples as equivocal. The Trep Sure test is approved by the US Food and Drug Administration (FDA; Silver Springs, MD, USA). Results of both ELISAs highly correlated with TPPA negative and positive samples. The analytical sensitivity of the Virotech Screen with international standards was determined at 0.02 IU/mL and 0.03 IU/mL, and was slightly superior to the Phoenix Trep-Sure.
The authors concluded that the Virotech Screen ELISA demonstrated good diagnostic sensitivity and specificity when evaluated as a screening test for syphilis among various patient populations, including samples with increased rates of false positive nontreponemal test results. The Virotech ELISA may be used in automatic analyzers as an alternative to the manual TPPA. However, the use of a confirmatory test remains a must in order to avoid false-positive results. The study was published in the May/June, 2013 issue of the journal Clinical Laboratory.
Related Links:
Sekisui Virotech
Zeus Scientific
Fujirebio
The etiological agent of syphilis, Treponema pallidum, cannot be cultured and there is no single optimal alternative test. Serological testing is the most frequently used approach in laboratory diagnosis of the disease.
Scientists at Sekisui Virotech (Rüsselsheim, Germany) compared their Treponema pallidum Screen ELISA with standard tests. These tests included the fluorescent treponemal antibody absorption (FTA-ABS, Zeus Scientific; Branchburg, NJ, USA) test, which is an indirect fluorescent antibody technique; the T. pallidum particle agglutination (TPPA, Fujirebio; Hoofddorp, the Netherlands) test, which is a qualitative assay for the detection of antibodies to T. pallidum in serum or plasma. The most relevant test used for comparison was the Trep-Sure ELISA (Phoenix Bio-Tech Corporation; Mississauga, ON, Canada).
To establish the sensitivity and specificity of the Virotech Screen, 421 serum samples from different panels of infected and non-infected patients, sera from seronegative pregnant women as well as international syphilis standard sera and panels were tested. In comparison to combined TPPA/FTA-abs tests, Phoenix Trep-Sure and Virotech Screen demonstrated a sensitivity of 100% and a specificity of 93.9% and 98.3%, respectively.
All samples of a well-defined syphilis serum panel were correctly identified by the Virotech test, whereas the Phoenix test identified two Treponema negative samples as equivocal. The Trep Sure test is approved by the US Food and Drug Administration (FDA; Silver Springs, MD, USA). Results of both ELISAs highly correlated with TPPA negative and positive samples. The analytical sensitivity of the Virotech Screen with international standards was determined at 0.02 IU/mL and 0.03 IU/mL, and was slightly superior to the Phoenix Trep-Sure.
The authors concluded that the Virotech Screen ELISA demonstrated good diagnostic sensitivity and specificity when evaluated as a screening test for syphilis among various patient populations, including samples with increased rates of false positive nontreponemal test results. The Virotech ELISA may be used in automatic analyzers as an alternative to the manual TPPA. However, the use of a confirmatory test remains a must in order to avoid false-positive results. The study was published in the May/June, 2013 issue of the journal Clinical Laboratory.
Related Links:
Sekisui Virotech
Zeus Scientific
Fujirebio
Latest Immunology News
- Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
- Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
- New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
- Routine Blood Test Can Predict Who Benefits Most from CAR T-Cell Therapy
- New Test Distinguishes Vaccine-Induced False Positives from Active HIV Infection
- Gene Signature Test Predicts Response to Key Breast Cancer Treatment
- Chip Captures Cancer Cells from Blood to Help Select Right Breast Cancer Treatment
- Blood-Based Liquid Biopsy Model Analyzes Immunotherapy Effectiveness
- Signature Genes Predict T-Cell Expansion in Cancer Immunotherapy
- Molecular Microscope Diagnostic System Assesses Lung Transplant Rejection
- Blood Test Tracks Treatment Resistance in High-Grade Serous Ovarian Cancer
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Simple One-Hour Saliva Test Detects Common Cancers
Early detection is critical for improving cancer outcomes, yet many diagnostic tests rely on invasive procedures such as blood draws or biopsies. Researchers are exploring simpler approaches that could... Read more
Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
Chemotherapy is often highly effective for germ cell tumors, but in a subset of patients, the disease does not respond well to standard treatment. For these individuals, doctors may consider high-dose... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreMicrobiology
view channel
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read more
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read morePathology
view channel
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read more
World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
Blood sampling is one of the most common clinical procedures, but it can be difficult or uncomfortable for many patients, especially older adults or individuals with certain medical conditions.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more






















