POC Diagnostic Platform Performs Immune Analysis Using One Drop of Fingertip Blood
Posted on 06 Jun 2025
As new COVID-19 variants continue to emerge and individuals accumulate complex histories of vaccination and infection, there is an urgent need for diagnostic tools that can quickly and accurately assess immune protection. Conventional methods require large blood samples and lengthy processing times. Now, researchers have developed a compact diagnostic platform, known as the Tip Optofluidic Immunoassay (TOI), that can evaluate antibody protection using just a single microliter of fingertip blood, delivering results in only 40 minutes.
The TOI platform was developed by scientists at Shenzhen Institutes of Advanced Technology (SIAT, Guangdong, China) and the Chinese Academy of Sciences (CAS, Beijing, China), in collaboration with multiple research partners. The initiative brings together expertise in biosensing, microfluidics, and synthetic biology to meet the growing need for accessible, field-ready diagnostic solutions. TOI works by integrating microfluidic biosensing with chemiluminescence detection in a portable format. At its core are high-affinity polystyrene immuno-reactors, which interface directly with standard pipette tips. These reactors are used in tandem with a portable chemiluminescent imaging station, enabling rapid and quantitative measurement of IgG binding levels, binding kinetics, and virus inhibition—all from a tiny drop of fingertip blood. The system operates without the need for complex lab equipment or high sample volumes.
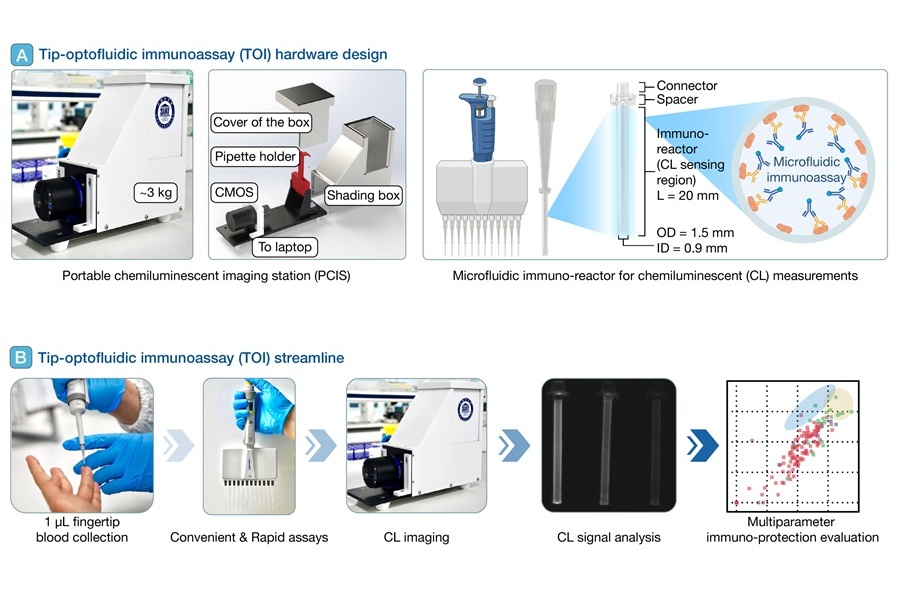
Compared to standard assays like ELISA or virus neutralization tests (VNTs), TOI significantly reduces both processing time and operational complexity. It delivers detection sensitivity at around 0.1 ng/mL, spans a dynamic range of 3 to 4.5 orders of magnitude, and achieves a signal-to-noise ratio of over 10,000. One of the platform’s key innovations is RIVIA 2.0, a rapid in vitro inhibition assay designed to simulate viral neutralization. Using rationally engineered SARS-CoV-2 spike ectodomain (S-ECD) trimers tagged with orientation-specific Avi-biotin, the system achieves precise and reproducible antibody activity measurements in just 20 minutes. The system was validated using 135 blood samples from 113 individuals, with a subset monitored over six months. TOI successfully identified broad-spectrum, high-titer antibody responses, especially in individuals with hybrid vaccine regimens.
Researchers also proposed a preliminary IgG concentration threshold of ~20 ng/mL, potentially linked to reduced short-term infection risk, indicating the test’s potential for predictive immune monitoring. Beyond COVID-19, the platform’s low sample requirement and portability make it suitable for other infectious diseases such as influenza and hepatitis, and for deployment in remote or resource-limited settings. TOI also offers valuable applications in therapeutic antibody development and vaccine efficacy studies, making it a versatile tool for both clinical and research use. This study demonstrates how TOI bridges the gap between advanced laboratory diagnostics and real-world application. By combining speed, sensitivity, and multi-dimensional immune assessment in a highly compact format, TOI sets a new benchmark for personalized and scalable infectious disease management.














