NMR-Based Method Measures Circulating Blood Citrate Levels
|
By LabMedica International staff writers Posted on 31 Mar 2021 |

Image: The Vantera Clinical Analyzer based on nuclear magnetic resonance (Photo courtesy of Liposcience)
Recent studies show that citrate is involved in several biological processes such as inflammation, cancer, insulin secretion, acetylation of histones, neurological development and hydroxylglutaric aciduria, indicating that it has functions beyond energy regulation.
Citrate associations with glaucoma, non-alcoholic fatty liver disease (NAFLD), bone disease and mortality have been observed. Monitoring circulating citrate could potentially be a diagnostic tool. While at present, urinary citrate is commonly used as a risk factor in kidney stone formation, serum/plasma citrate is scarcely utilized for disease diagnosis or prognosis.
Laboratorians at the Laboratory Corporation of America Holdings (Labcorp, Morrisville, NC, USA) took blood samples from volunteers in Greiner tubes allowed to clot (30 minutes) in an upright position and centrifuged (3,000 rpm, 10-15 minutes) immediately after clotting. Samples collected into plain red-top tubes and BD Gel Barrier serum tube (Becton Dickinson and Company, Franklin Lakes, NJ, USA) were held upright (red-top tubes for 45 minutes; BD Gel Barrier tubes for 30 minutes) at room temperature to clot and were promptly centrifuged.
Sample preparation (i.e., 1:1 (v/v) dilution of serum or plasma with phosphate buffer) was performed automatically on the Vantera Clinical Analyzer (Liposcience, Raleigh, NC). One-dimensional 1H NMR spectra were collected on a 400 MHz spectrometers at 47 °C. WET was used to suppress the water signal. The total acquisition time for each spectrum was 48 seconds. The NMR instruments are calibrated using 15 mM trimethyl acetic acid as a calibrator and reference standard to verify instrument performance on a daily basis. A restricted region of the collected spectrum, where the four citrate resonances appear, was used for quantification. To determine if the assay has adequate sensitivity to measure clinically relevant concentrations of citrate, the assay was used to quantify citrate in 533 apparently healthy adults, and in the general population (n=133,567).
The team reported that the limit of quantification (LOQ) for the assay was determined to be 1.48 mg/dL. Linearity was demonstrated over a wide range of concentrations (1.40 to 4.46 mg/dL). Coefficients of variation (%CV) for intra- and inter-assay precision ranged from 5.8-9.3 and 5.2-9.6%, respectively. Substances tested did not elicit interference with assay results. Specimen type comparison revealed <1% bias between serum and plasma samples, except for heparin plasma (3% bias). Stability was demonstrated up to eight days at room temperature and longer at lower temperatures. In a cohort of apparently healthy adults, the reference interval was <1.48 to 2.97 mg/dL. Slightly higher values were observed in the general population.
The authors concluded that the newly developed NMR-based assay exhibits analytical characteristics that allow the accurate quantification of clinically relevant citrate concentrations. The assay provides a simple and fast means to analyze samples for clinical and other studies. The study was published on March 18, 2021 in the journal Practical Laboratory Medicine.
Related Links:
Laboratory Corporation of America Holdings
Becton Dickinson and Company
Liposcience
Citrate associations with glaucoma, non-alcoholic fatty liver disease (NAFLD), bone disease and mortality have been observed. Monitoring circulating citrate could potentially be a diagnostic tool. While at present, urinary citrate is commonly used as a risk factor in kidney stone formation, serum/plasma citrate is scarcely utilized for disease diagnosis or prognosis.
Laboratorians at the Laboratory Corporation of America Holdings (Labcorp, Morrisville, NC, USA) took blood samples from volunteers in Greiner tubes allowed to clot (30 minutes) in an upright position and centrifuged (3,000 rpm, 10-15 minutes) immediately after clotting. Samples collected into plain red-top tubes and BD Gel Barrier serum tube (Becton Dickinson and Company, Franklin Lakes, NJ, USA) were held upright (red-top tubes for 45 minutes; BD Gel Barrier tubes for 30 minutes) at room temperature to clot and were promptly centrifuged.
Sample preparation (i.e., 1:1 (v/v) dilution of serum or plasma with phosphate buffer) was performed automatically on the Vantera Clinical Analyzer (Liposcience, Raleigh, NC). One-dimensional 1H NMR spectra were collected on a 400 MHz spectrometers at 47 °C. WET was used to suppress the water signal. The total acquisition time for each spectrum was 48 seconds. The NMR instruments are calibrated using 15 mM trimethyl acetic acid as a calibrator and reference standard to verify instrument performance on a daily basis. A restricted region of the collected spectrum, where the four citrate resonances appear, was used for quantification. To determine if the assay has adequate sensitivity to measure clinically relevant concentrations of citrate, the assay was used to quantify citrate in 533 apparently healthy adults, and in the general population (n=133,567).
The team reported that the limit of quantification (LOQ) for the assay was determined to be 1.48 mg/dL. Linearity was demonstrated over a wide range of concentrations (1.40 to 4.46 mg/dL). Coefficients of variation (%CV) for intra- and inter-assay precision ranged from 5.8-9.3 and 5.2-9.6%, respectively. Substances tested did not elicit interference with assay results. Specimen type comparison revealed <1% bias between serum and plasma samples, except for heparin plasma (3% bias). Stability was demonstrated up to eight days at room temperature and longer at lower temperatures. In a cohort of apparently healthy adults, the reference interval was <1.48 to 2.97 mg/dL. Slightly higher values were observed in the general population.
The authors concluded that the newly developed NMR-based assay exhibits analytical characteristics that allow the accurate quantification of clinically relevant citrate concentrations. The assay provides a simple and fast means to analyze samples for clinical and other studies. The study was published on March 18, 2021 in the journal Practical Laboratory Medicine.
Related Links:
Laboratory Corporation of America Holdings
Becton Dickinson and Company
Liposcience
Latest Clinical Chem. News
- 3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
- POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
- Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
- New Blood Testing Method Detects Potent Opioids in Under Three Minutes
- Wireless Hepatitis B Test Kit Completes Screening and Data Collection in One Step
- Pain-Free, Low-Cost, Sensitive, Radiation-Free Device Detects Breast Cancer in Urine
- Spit Test Detects Breast Cancer in Five Seconds
- Electrochemical Sensors with Next-Generation Coating Advances Precision Diagnostics at POC
- First-Of-Its-Kind Handheld Device Accurately Detects Fentanyl in Urine within Seconds
- New Fluorescent Sensor Array Lights up Alzheimer’s-Related Proteins for Earlier Detection
- Automated Mass Spectrometry-Based Clinical Analyzer Could Transform Lab Testing
- Highly Sensitive pH Sensor to Aid Detection of Cancers and Vector-Borne Viruses
- Non-Invasive Sensor Monitors Changes in Saliva Compositions to Rapidly Diagnose Diabetes
- Breakthrough Immunoassays to Aid in Risk Assessment of Preeclampsia
- Urine Test for Monitoring Changes in Kidney Health Markers Can Predict New-Onset Heart Failure
- AACC Releases Comprehensive Diabetes Testing Guidelines
Channels
Clinical Chemistry
view channel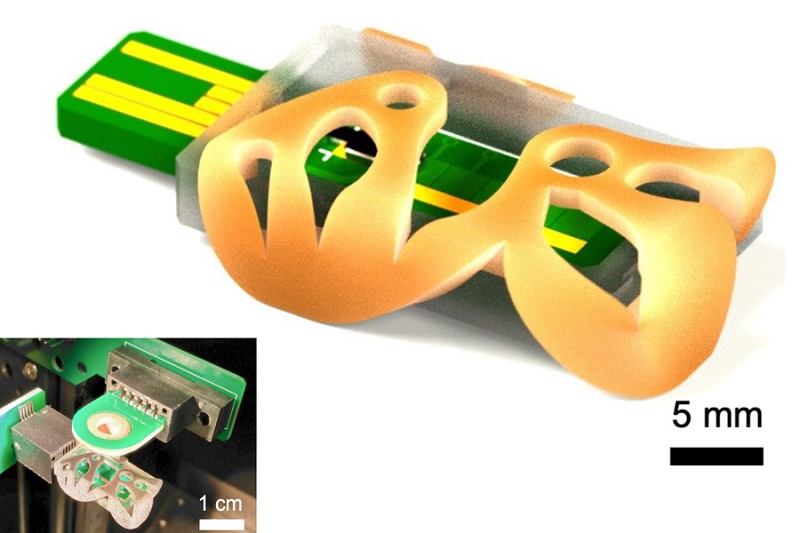
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more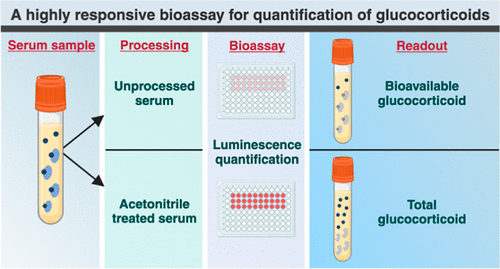
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channelBlood Proteins Could Warn of Cancer Seven Years before Diagnosis
Two studies have identified proteins in the blood that could potentially alert individuals to the presence of cancer more than seven years before the disease is clinically diagnosed. Researchers found... Read moreUltrasound-Aided Blood Testing Detects Cancer Biomarkers from Cells
Ultrasound imaging serves as a noninvasive method to locate and monitor cancerous tumors effectively. However, crucial details about the cancer, such as the specific types of cells and genetic mutations... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more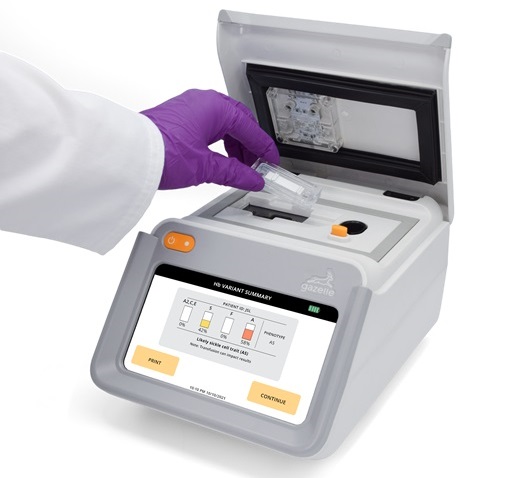
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel.jpg)
AI Predicts Tumor-Killing Cells with High Accuracy
Cellular immunotherapy involves extracting immune cells from a patient's tumor, potentially enhancing their cancer-fighting capabilities through engineering, and then expanding and reintroducing them into the body.... Read more
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channelHyperspectral Dark-Field Microscopy Enables Rapid and Accurate Identification of Cancerous Tissues
Breast cancer remains a major cause of cancer-related mortality among women. Breast-conserving surgery (BCS), also known as lumpectomy, is the removal of the cancerous lump and a small margin of surrounding tissue.... Read more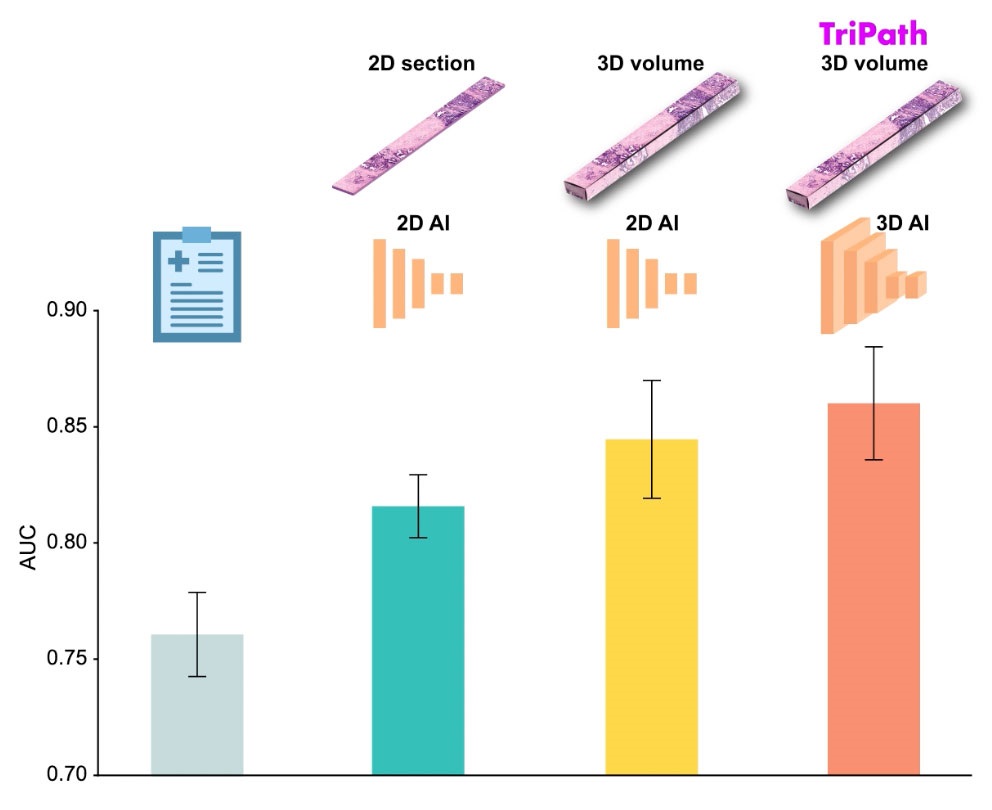
AI Advancements Enable Leap into 3D Pathology
Human tissue is complex, intricate, and naturally three-dimensional. However, the thin two-dimensional tissue slices commonly used by pathologists to diagnose diseases provide only a limited view of the... Read more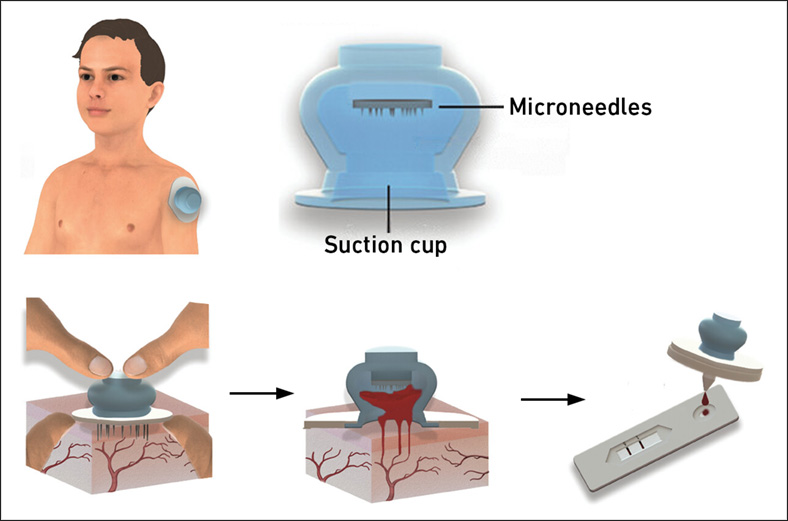
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more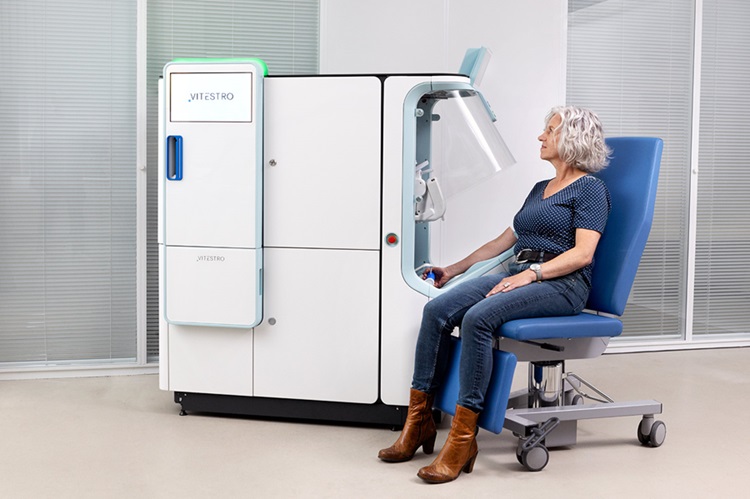
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read moreIndustry
view channel
Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
Unlike severe traumatic brain injury (TBI), mild TBI often does not show clear correlations with abnormalities detected through head computed tomography (CT) scans. Consequently, there is a pressing need... Read more
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)











_1.jpg)
.jpg)
