New Microarray to Boost Developmental Delay Research
|
By LabMedica International staff writers Posted on 23 Jun 2015 |
A new tool for studying the genetic causes of developmental delay was unveiled at a recent human genetics conference.
Despite three decades of successful, predominantly phenotype-driven discovery of the genetic causes of monogenic disorders, up to half of children with severe developmental disorders of probable genetic origin remain without a genetic diagnosis. Particularly challenging are those disorders rare enough to have eluded recognition as a discrete clinical entity, those with highly variable clinical manifestations, and those that are difficult to distinguish from other, very similar, disorders.
A new tool for studying the genetic foundation of developmental delay, the Oxford Gene Technology (Oxford, United Kingdom) Cytosure Constitutional v3 DNA microarray, was introduced to genomic researchers at the June 2015 European Society of Human Genetics (ESHG) Conference held in Glasgow (United Kingdom).
The Cytosure Constitutional v3 array was developed in collaboration with the Wellcome Trust Sanger Institute (Hinxton, United Kingdom). This tool combines the most up-to-date and relevant developmental delay content from the recent Deciphering Developmental Disorders (DDD) study with the latest updates from ClinGen, an international cooperative dedicated to sharing genomic and phenotypic developmental disorder data provided by clinicians, researchers, and patients through centralized databases for clinical and research use.
Oxford Gene Technology has optimized the arrays via a proprietary probe design algorithm and experimental validation, enabling the selection of highly-targeted, specific probes throughout the genome. Regions with the highest priority are covered at exon-level resolution on the arrays, enabling single-exon detection in up to 502 prioritized genes of interest.
James Clough, executive vice president commercial at Oxford Gene Technology, said, “Through combining our superior array design capabilities with the latest research-led gene content, we are proud to offer our customers the most advanced array design available for accurately and easily identifying the causal aberrations underlying developmental delay. These new products underline Oxford Gene Technology’s long-standing commitment to providing cytogenetics researchers with the latest tools to further understand developmental disorders.”
Related Links:
Oxford Gene Technology
Wellcome Trust Sanger Institute
ClinGen
Despite three decades of successful, predominantly phenotype-driven discovery of the genetic causes of monogenic disorders, up to half of children with severe developmental disorders of probable genetic origin remain without a genetic diagnosis. Particularly challenging are those disorders rare enough to have eluded recognition as a discrete clinical entity, those with highly variable clinical manifestations, and those that are difficult to distinguish from other, very similar, disorders.
A new tool for studying the genetic foundation of developmental delay, the Oxford Gene Technology (Oxford, United Kingdom) Cytosure Constitutional v3 DNA microarray, was introduced to genomic researchers at the June 2015 European Society of Human Genetics (ESHG) Conference held in Glasgow (United Kingdom).
The Cytosure Constitutional v3 array was developed in collaboration with the Wellcome Trust Sanger Institute (Hinxton, United Kingdom). This tool combines the most up-to-date and relevant developmental delay content from the recent Deciphering Developmental Disorders (DDD) study with the latest updates from ClinGen, an international cooperative dedicated to sharing genomic and phenotypic developmental disorder data provided by clinicians, researchers, and patients through centralized databases for clinical and research use.
Oxford Gene Technology has optimized the arrays via a proprietary probe design algorithm and experimental validation, enabling the selection of highly-targeted, specific probes throughout the genome. Regions with the highest priority are covered at exon-level resolution on the arrays, enabling single-exon detection in up to 502 prioritized genes of interest.
James Clough, executive vice president commercial at Oxford Gene Technology, said, “Through combining our superior array design capabilities with the latest research-led gene content, we are proud to offer our customers the most advanced array design available for accurately and easily identifying the causal aberrations underlying developmental delay. These new products underline Oxford Gene Technology’s long-standing commitment to providing cytogenetics researchers with the latest tools to further understand developmental disorders.”
Related Links:
Oxford Gene Technology
Wellcome Trust Sanger Institute
ClinGen
Read the full article by registering today, it's FREE! 

Register now for FREE to LabMedica.com and get complete access to news and events that shape the world of Clinical Laboratory Medicine. 
- Free digital version edition of LabMedica International sent by email on regular basis
- Free print version of LabMedica International magazine (available only outside USA and Canada).
- Free and unlimited access to back issues of LabMedica International in digital format
- Free LabMedica International Newsletter sent every week containing the latest news
- Free breaking news sent via email
- Free access to Events Calendar
- Free access to LinkXpress new product services
- REGISTRATION IS FREE AND EASY!
Sign in: Registered website members
Sign in: Registered magazine subscribers
Latest BioResearch News
- Genome Analysis Predicts Likelihood of Neurodisability in Oxygen-Deprived Newborns
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- Gene Fusion Protein Proposed as Prostate Cancer Biomarker
- NIV Test to Diagnose and Monitor Vascular Complications in Diabetes
- Semen Exosome MicroRNA Proves Biomarker for Prostate Cancer
- Genetic Loci Link Plasma Lipid Levels to CVD Risk
- Newly Identified Gene Network Aids in Early Diagnosis of Autism Spectrum Disorder
- Link Confirmed between Living in Poverty and Developing Diseases
- Genomic Study Identifies Kidney Disease Loci in Type I Diabetes Patients
- Liquid Biopsy More Effective for Analyzing Tumor Drug Resistance Mutations
- New Liquid Biopsy Assay Reveals Host-Pathogen Interactions
- Method Developed for Enriching Trophoblast Population in Samples
Channels
Clinical Chemistry
view channel
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more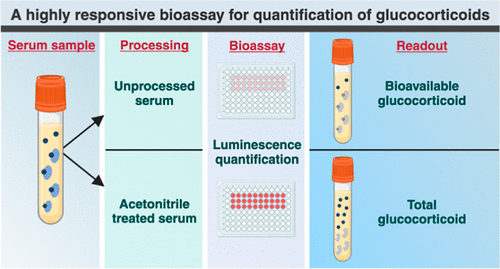
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
RNA-Powered Molecular Test to Help Combat Early-Age Onset Colorectal Cancer
Colorectal cancer (CRC) ranks as the second most lethal cancer in the United States. Nevertheless, many Americans eligible for screening do not undergo testing due to limited access or reluctance towards... Read more
Advanced Blood Test to Spot Alzheimer's Before Progression to Dementia
Alzheimer’s disease is well known for its slow development over many years, which typically leads to treatment interventions only after the disease has advanced to stages where it may be nearly impossible... Read more
Multi-Omic Noninvasive Urine-Based DNA Test to Improve Bladder Cancer Detection
Hematuria, the presence of blood in urine, is commonly associated with bladder cancer and is its most frequent symptom. Current guidelines recommend referring patients with hematuria to urology for clinical... Read more.jpg)
First of Its Kind NGS Assay for Precise Detection of BCR::ABL1 Fusion Gene to Enable Personalized Leukemia Treatment
The BCR::ABL1 fusion gene plays a key role in the pathogenesis of several blood cancers, particularly chronic myeloid leukemia (CML). This gene results from a chromosomal translocation that causes constitutive... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channel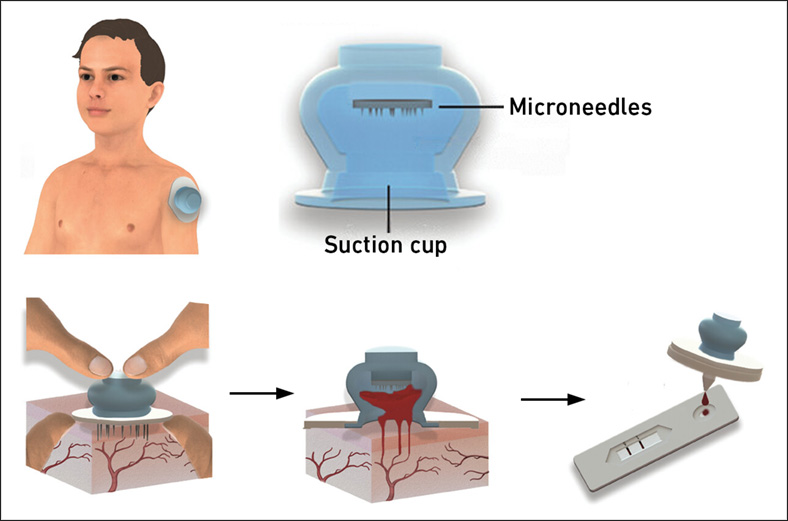
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more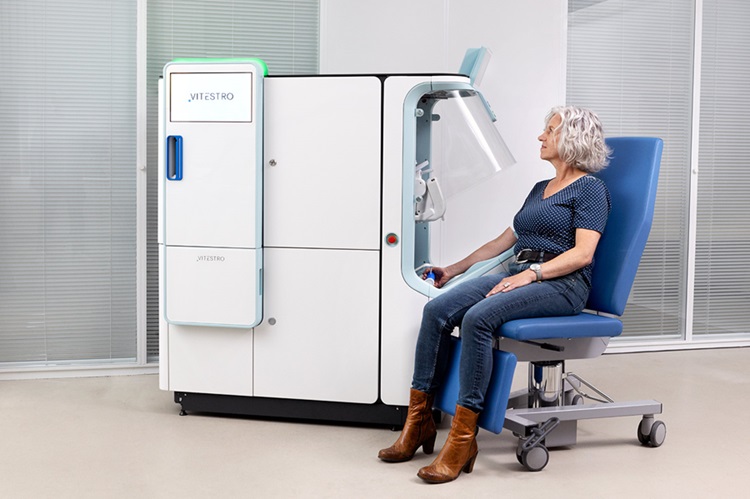
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read more.jpg)
Use of DICOM Images for Pathology Diagnostics Marks Significant Step towards Standardization
Digital pathology is rapidly becoming a key aspect of modern healthcare, transforming the practice of pathology as laboratories worldwide adopt this advanced technology. Digital pathology systems allow... Read more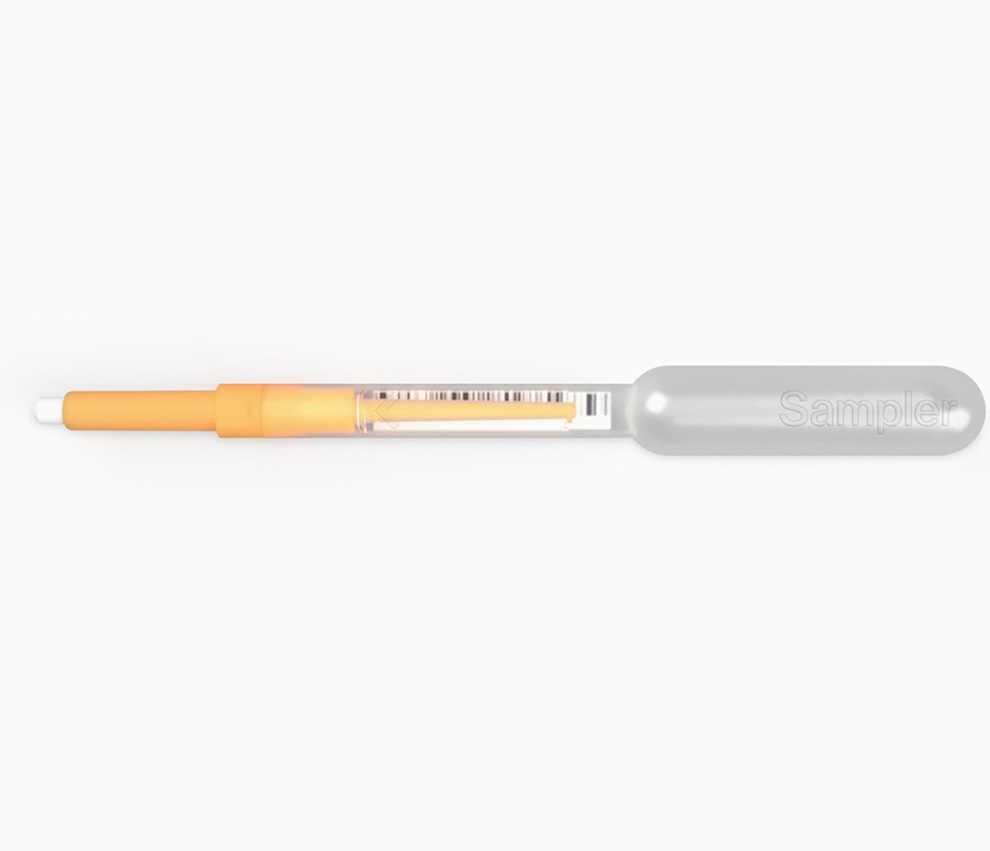
First of Its Kind Universal Tool to Revolutionize Sample Collection for Diagnostic Tests
The COVID pandemic has dramatically reshaped the perception of diagnostics. Post the pandemic, a groundbreaking device that combines sample collection and processing into a single, easy-to-use disposable... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more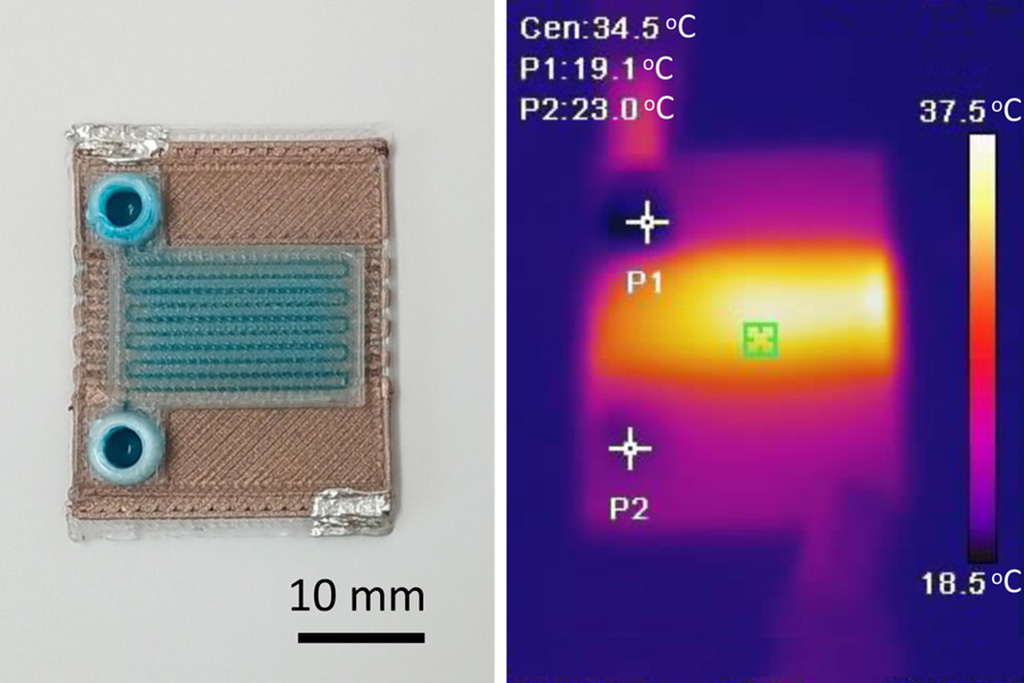
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)
Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
Thermo Fisher Scientific (Waltham, MA USA) has entered into a strategic distribution agreement with Bio-Techne Corporation (Minneapolis, MN, USA), resulting in a significant collaboration between two industry... Read more
ECCMID Congress Name Changes to ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland) has evolved remarkably. The society is now stronger and broader than ever before... Read more