Developing POC Tests for Alzheimer’s to Improve Monitoring and Management of the Disease
|
By LabMedica International staff writers Posted on 22 Mar 2016 |
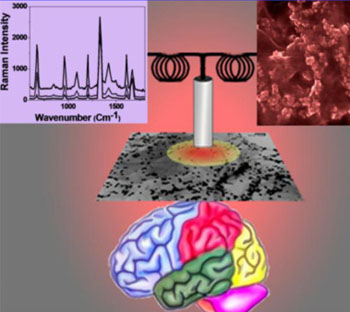
Image: Illustration of a protocol showing a pathway to develop core-shell nanoparticle/hybrid graphene oxide based multi-functional platform label-free SERS detection of β-amyloid toward developing a portable point-of-care blood test to monitor Alzheimer’s disease progression (Figure courtesy of Teresa Demeritt et al., 2015, ACS Appl. Mater. Interfaces; Copyright ACS-2015).
Toward development of a portable point-of-care (POC) biosensor for Alzheimer’s disease (AD), a new review explores recent advancements in nano-enabling electrochemical beta-amyloid (β-A) -sensing technologies. A simple, rapid POC biomarker test could greatly improve AD management and personalized treatment, also in developing countries.
The authors of the review, from the College of Medicine at Florida International University (Miami, FL, USA), are taking a new approach to diagnosing AD: measuring β-A in the blood with a POC test. AD is caused by high levels of β-A in the brain that lead to degeneration of brain cells. Various types of scans and immunoassays, such as MRI and ELISA, are available to estimate β-A levels in the brain. But the peptide can also be found at lower levels in blood, making it a useful biomarker for a simple test.
Currently there is no sensitive or inexpensive way to measure β-A levels in blood samples. The authors of the new review plan to change that. “We want to develop a point-of-care system where a small drop of blood plasma can reveal their β-A level immediately so that a doctor can tailor a patient’s therapy immediately,” said lead author Dr. Ajeet Kaushik, “The drugs used to treat AD can have side effects, so it’s better for patients not to overdose. With the right data, doctors can respond quickly to changes in a patient’s brain by reducing or increasing their dose.”
In the review, Dr. Kaushik and colleagues looked at each of the methods available to measure β-A concentration in brain tissue and in blood. None of the existing tests can be done at the bedside and all need special expertise and large samples. They also take a long time to generate a useful result—the main existing test, an ELISA, takes 6–8 hours. In comparison, the cheap, simple biosensor Dr. Kaushik and colleagues describe can measure β-A in the blood at low (pico molar) concentrations in just 30 minutes.
“Even though existing technologies are well established, we need to move towards small sample, high accuracy tests that can be used in all environments, from developed countries to rural settings. Our goal is to develop a test that’s sensitive, small, and affordable,” said Dr. Kaushik. To develop the new biosensor, the team will need many bio-fluid samples taken at different stages of the disease. Finding these samples will be challenging, but the review demonstrates that a biosensor is achievable. Such a test would also “show if and when the disease reaches an untreatable level. In the future we hope a rapid biosensor test for AD will help scientists study disease progression and help clinicians deliver personalized therapy to patients.”
The study, by Kaushik A et al., was published online ahead of print January 28, 2016, in the journal Biosensors and Bioelectronics.
Related Links:
Florida International University
The authors of the review, from the College of Medicine at Florida International University (Miami, FL, USA), are taking a new approach to diagnosing AD: measuring β-A in the blood with a POC test. AD is caused by high levels of β-A in the brain that lead to degeneration of brain cells. Various types of scans and immunoassays, such as MRI and ELISA, are available to estimate β-A levels in the brain. But the peptide can also be found at lower levels in blood, making it a useful biomarker for a simple test.
Currently there is no sensitive or inexpensive way to measure β-A levels in blood samples. The authors of the new review plan to change that. “We want to develop a point-of-care system where a small drop of blood plasma can reveal their β-A level immediately so that a doctor can tailor a patient’s therapy immediately,” said lead author Dr. Ajeet Kaushik, “The drugs used to treat AD can have side effects, so it’s better for patients not to overdose. With the right data, doctors can respond quickly to changes in a patient’s brain by reducing or increasing their dose.”
In the review, Dr. Kaushik and colleagues looked at each of the methods available to measure β-A concentration in brain tissue and in blood. None of the existing tests can be done at the bedside and all need special expertise and large samples. They also take a long time to generate a useful result—the main existing test, an ELISA, takes 6–8 hours. In comparison, the cheap, simple biosensor Dr. Kaushik and colleagues describe can measure β-A in the blood at low (pico molar) concentrations in just 30 minutes.
“Even though existing technologies are well established, we need to move towards small sample, high accuracy tests that can be used in all environments, from developed countries to rural settings. Our goal is to develop a test that’s sensitive, small, and affordable,” said Dr. Kaushik. To develop the new biosensor, the team will need many bio-fluid samples taken at different stages of the disease. Finding these samples will be challenging, but the review demonstrates that a biosensor is achievable. Such a test would also “show if and when the disease reaches an untreatable level. In the future we hope a rapid biosensor test for AD will help scientists study disease progression and help clinicians deliver personalized therapy to patients.”
The study, by Kaushik A et al., was published online ahead of print January 28, 2016, in the journal Biosensors and Bioelectronics.
Related Links:
Florida International University
Latest Pathology News
- Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
- Molecular Imaging to Reduce Need for Melanoma Biopsies
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
- First-Of-Its-Kind Test Identifies Autism Risk at Birth
- AI Algorithms Improve Genetic Mutation Detection in Cancer Diagnostics
- Skin Biopsy Offers New Diagnostic Method for Neurodegenerative Diseases
- Fast Label-Free Method Identifies Aggressive Cancer Cells
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
New Blood Test Can Help Predict Testicular Cancer Recurrence
Stage 1 testicular germ cell tumor is typically treated with surgery followed by active surveillance. Although most patients experience strong long-term outcomes, about one in four will see their cancer... Read more
New Test Detects Alzheimer’s by Analyzing Altered Protein Shapes in Blood
Alzheimer’s disease begins developing years before memory loss or other symptoms become visible. Misfolded proteins gradually accumulate in the brain, disrupting normal cellular processes.... Read more
New Diagnostic Markers for Multiple Sclerosis Discovered in Cerebrospinal Fluid
Multiple sclerosis (MS) affects nearly three million people worldwide and can cause symptoms such as numbness, visual disturbances, fatigue, and neurological disability. Diagnosing the disease can be challenging... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreMicrobiology
view channel
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read more
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read morePathology
view channel
Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
Polymerase Chain Reaction (PCR) testing became widely recognized during the COVID-19 pandemic as a powerful method for detecting viruses such as SARS-CoV-2. PCR belongs to a group of diagnostic methods... Read more
Molecular Imaging to Reduce Need for Melanoma Biopsies
Melanoma is the deadliest form of skin cancer and accounts for the vast majority of skin cancer-related deaths. Because early melanomas can closely resemble benign moles, clinicians often rely on visual... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more





















