Multianalyte Test Predicts Drug Resistance in Esophageal Cancer
|
By LabMedica International staff writers Posted on 16 Mar 2015 |
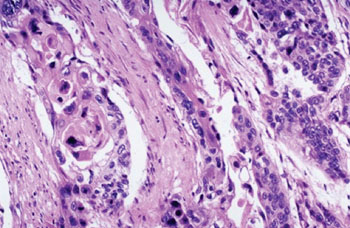
Image: Histopathology of esophageal carcinoma showing infiltrating nests of neoplastic cells (Photo courtesy of Dr. Elliot Weisenberg, MD).
A multianalyte algorithmic immunohistochemistry (IHC) assay accurately identifies patients with locoregional esophageal adenocarcinoma (EC) who exhibit extreme resistance to neoadjuvant chemoradiotherapy.
The test analyzes the localization of three protein biomarkers within a patient's tumor to classify the cancer as either responsive to or resistant to presurgical chemoradiotherapy and demonstrates strong accuracy and specificity in identifying patients with tumors that are unlikely to respond to standard presurgical (neoadjuvant) chemotherapy and radiation.
Scientists at Baylor College of Medicine (Houston TX, USA) and their colleagues studied archived biopsy specimens of EC which were subject to IHC examination of compartmentalized immunoreactivity of nuclear factor kappa B (NF-κB), Sonic Hedgehog (SHH), and GLI family zinc finger 1 (Gli-1), and a labeling index score was assigned to each biomarker. Pretreatment tumor biopsies were used to evaluate resistance (exCTRT) or responsiveness to (non-exCTRT) standard presurgical chemoradiotherapy (CTRT) regimens under accredited certified laboratory protocols.
According to validation studies, the DecisionDx-EC test (Castle Biosciences, Inc.; Friendswood, TX, USA) can reliably differentiate patients who are complete or partial responders to chemoradiotherapy from those who are non-responders. An initial, single center clinical validation study of 167 patients, which was used as training set for the current validation study, achieved an area under the curve (AUC) of 0.96 and an overall accuracy of 90%. The second validation, enrolled 64 patients from two independent institutions, and achieved an AUC of 0.96 and an overall accuracy of 84% for classifying which patients are likely to be highly resistant to presurgical chemotherapy treatment for esophageal cancer.
Derek Maetzold, BS, MBA, the President and CEO of Castle Biosciences, said, “Publication of these results is a culmination of our extensive program to analytically and clinically validate a new predictive test for esophageal cancer. DecisionDx-EC fits well within our strategy of developing and commercializing valuable prognostic tests that help physicians to select the most appropriate care for their patients.” The study was published on February 19, 2015, in the journal Gastrointestinal Cancer: Targets and Therapy.
Related Links:
Baylor College of Medicine
Castle Biosciences, Inc.
The test analyzes the localization of three protein biomarkers within a patient's tumor to classify the cancer as either responsive to or resistant to presurgical chemoradiotherapy and demonstrates strong accuracy and specificity in identifying patients with tumors that are unlikely to respond to standard presurgical (neoadjuvant) chemotherapy and radiation.
Scientists at Baylor College of Medicine (Houston TX, USA) and their colleagues studied archived biopsy specimens of EC which were subject to IHC examination of compartmentalized immunoreactivity of nuclear factor kappa B (NF-κB), Sonic Hedgehog (SHH), and GLI family zinc finger 1 (Gli-1), and a labeling index score was assigned to each biomarker. Pretreatment tumor biopsies were used to evaluate resistance (exCTRT) or responsiveness to (non-exCTRT) standard presurgical chemoradiotherapy (CTRT) regimens under accredited certified laboratory protocols.
According to validation studies, the DecisionDx-EC test (Castle Biosciences, Inc.; Friendswood, TX, USA) can reliably differentiate patients who are complete or partial responders to chemoradiotherapy from those who are non-responders. An initial, single center clinical validation study of 167 patients, which was used as training set for the current validation study, achieved an area under the curve (AUC) of 0.96 and an overall accuracy of 90%. The second validation, enrolled 64 patients from two independent institutions, and achieved an AUC of 0.96 and an overall accuracy of 84% for classifying which patients are likely to be highly resistant to presurgical chemotherapy treatment for esophageal cancer.
Derek Maetzold, BS, MBA, the President and CEO of Castle Biosciences, said, “Publication of these results is a culmination of our extensive program to analytically and clinically validate a new predictive test for esophageal cancer. DecisionDx-EC fits well within our strategy of developing and commercializing valuable prognostic tests that help physicians to select the most appropriate care for their patients.” The study was published on February 19, 2015, in the journal Gastrointestinal Cancer: Targets and Therapy.
Related Links:
Baylor College of Medicine
Castle Biosciences, Inc.
Latest Pathology News
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
- First-Of-Its-Kind Test Identifies Autism Risk at Birth
- AI Algorithms Improve Genetic Mutation Detection in Cancer Diagnostics
- Skin Biopsy Offers New Diagnostic Method for Neurodegenerative Diseases
- Fast Label-Free Method Identifies Aggressive Cancer Cells
- New X-Ray Method Promises Advances in Histology
- Single-Cell Profiling Technique Could Guide Early Cancer Detection
- Intraoperative Tumor Histology to Improve Cancer Surgeries
- Rapid Stool Test Could Help Pinpoint IBD Diagnosis
Channels
Clinical Chemistry
view channel
Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
Timely evaluation of cognitive symptoms in primary care is often limited by restricted access to specialized diagnostics and invasive confirmatory procedures. Clinicians need accessible tools to determine... Read more
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read moreMolecular Diagnostics
view channel
New Genome Sequencing Technique Measures Epstein-Barr Virus in Blood
The Epstein–Barr virus (EBV) infects up to 95% of adults worldwide and remains in the body for life. While usually kept under control, the virus is linked to cancers such as Hodgkin’s lymphoma and autoimmune... Read more
Blood Test Boosts Early Detection of Brain Cancer
Brain and central nervous system (CNS) tumors are often diagnosed at an advanced stage, when treatment options are limited, and survival rates remain low. Around 300,000 new cases are diagnosed each year... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Three-Test Panel Launched for Detection of Liver Fluke Infections
Parasitic liver fluke infections remain endemic in parts of Asia, where transmission commonly occurs through consumption of raw freshwater fish or aquatic plants. Chronic infection is a well-established... Read more
Rapid Test Promises Faster Answers for Drug-Resistant Infections
Drug-resistant pathogens continue to pose a growing threat in healthcare facilities, where delayed detection can impede outbreak control and increase mortality. Candida auris is notoriously difficult to... Read more
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreIndustry
view channel
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more








 (3) (1).png)