ERBA Diagnostics Enters Brazilian Sector with Leading-Edge Clinical Testing Portfolio
|
By LabMedica International staff writers Posted on 02 Nov 2014 |
ERBA Diagnostics, Inc. (Miami Lakes, FL, USA) has announced that an integrated portfolio of its latest in vitro diagnostics platforms and reagents are now being made available to hospitals, references labs, and physician clinics in Brazil. The product line includes a comprehensive suite of instruments, reagents, and kits for immunology, clinical chemistry, hematology, diabetes, and infectious disease testing, with an emphasis on hematology and clinical chemistry. ERBA Diagnostics intends to introduce this suite of products as the product registrations in Brazil are completed.
"We believe that our line of products is ideally suited to address the rapidly growing medical needs for Brazil," said Mohan Gopalkrishnan, chief executive officer of ERBA Diagnostics, "Within Brazil, there is a predominance of smaller, local healthcare providers. We expect that our proprietary instruments and reagents will allow these providers access to the latest diagnostics at a scale and price point that serves their needs. Our initial experience in Brazil during the past three months has been encouraging and we look forward to expanding the number of clinics and hospitals we serve, as we continue to register the full suite of our products in Brazil."
The estimated market for in vitro diagnostics products in Brazil was approximately USD 1.0 billion in 2012 and is expected to grow to USD 1.4 billion by 2017, far exceeding the rate of growth in the world market for in vitro diagnostics products. Brazil has a population of 200 million people and the country is undergoing a rapid expansion of the elderly and middle class. These factors are contributing to the increased demand for clinical testing at both public and private healthcare providers as a means to inform treatment, improve outcomes, and reduce overall healthcare expenditures.
"Given our commitment to Brazil, and as part of our continued effort to streamline our operations, we are evaluating opportunities to establish local manufacturing capabilities," added Mr. Gopalkrishnan, "We believe that establishing manufacturing capabilities in Brazil will ultimately allow us to operate as efficiently as possible while simultaneously positioning us to best serve the diagnostic testing needs of the country's healthcare providers."
Related Links:
ERBA Diagnostics
"We believe that our line of products is ideally suited to address the rapidly growing medical needs for Brazil," said Mohan Gopalkrishnan, chief executive officer of ERBA Diagnostics, "Within Brazil, there is a predominance of smaller, local healthcare providers. We expect that our proprietary instruments and reagents will allow these providers access to the latest diagnostics at a scale and price point that serves their needs. Our initial experience in Brazil during the past three months has been encouraging and we look forward to expanding the number of clinics and hospitals we serve, as we continue to register the full suite of our products in Brazil."
The estimated market for in vitro diagnostics products in Brazil was approximately USD 1.0 billion in 2012 and is expected to grow to USD 1.4 billion by 2017, far exceeding the rate of growth in the world market for in vitro diagnostics products. Brazil has a population of 200 million people and the country is undergoing a rapid expansion of the elderly and middle class. These factors are contributing to the increased demand for clinical testing at both public and private healthcare providers as a means to inform treatment, improve outcomes, and reduce overall healthcare expenditures.
"Given our commitment to Brazil, and as part of our continued effort to streamline our operations, we are evaluating opportunities to establish local manufacturing capabilities," added Mr. Gopalkrishnan, "We believe that establishing manufacturing capabilities in Brazil will ultimately allow us to operate as efficiently as possible while simultaneously positioning us to best serve the diagnostic testing needs of the country's healthcare providers."
Related Links:
ERBA Diagnostics
Latest Industry News
- QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
- WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- WHX Labs Dubai to Gather Global Experts in Antimicrobial Resistance at Inaugural AMR Leaders’ Summit
- BD and Penn Institute Collaborate to Advance Immunotherapy through Flow Cytometry
- Abbott Acquires Cancer-Screening Company Exact Sciences
- Roche and Freenome Collaborate to Develop Cancer Screening Tests
- Co-Diagnostics Forms New Business Unit to Develop AI-Powered Diagnostics
- Qiagen Acquires Single-Cell Omics Firm Parse Biosciences
- Puritan Medical Products Showcasing Innovation at AMP2025 in Boston
- Advanced Instruments Merged Under Nova Biomedical Name
- Bio-Rad and Biodesix Partner to Develop Droplet Digital PCR High Complexity Assays
- Hologic to be Acquired by Blackstone and TPG
- Bio-Techne and Oxford Nanopore to Accelerate Development of Genetics Portfolio
Channels
Clinical Chemistry
view channel
Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
Timely evaluation of cognitive symptoms in primary care is often limited by restricted access to specialized diagnostics and invasive confirmatory procedures. Clinicians need accessible tools to determine... Read more
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read moreMolecular Diagnostics
view channel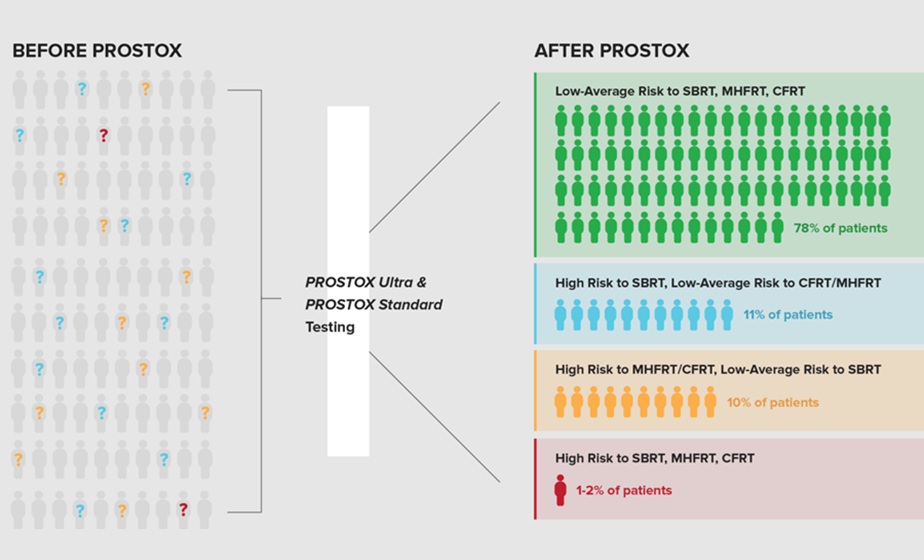
Genetic Test Predicts Radiation Therapy Risk for Prostate Cancer Patients
External beam radiation therapy is widely used to treat localized prostate cancer, which has a five-year survival rate exceeding 99%. However, more than 20% of patients develop persistent urinary side... Read more
Genetic Test Aids Early Detection and Improved Treatment for Cancers
Lynch syndrome is a hereditary genetic condition that significantly increases the risk of several cancers, including those of the bowel and urinary tract. Urinary tract cancers—affecting the kidney, bladder,... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Three-Test Panel Launched for Detection of Liver Fluke Infections
Parasitic liver fluke infections remain endemic in parts of Asia, where transmission commonly occurs through consumption of raw freshwater fish or aquatic plants. Chronic infection is a well-established... Read more
Rapid Test Promises Faster Answers for Drug-Resistant Infections
Drug-resistant pathogens continue to pose a growing threat in healthcare facilities, where delayed detection can impede outbreak control and increase mortality. Candida auris is notoriously difficult to... Read more
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
Urine testing is a critical, non-invasive diagnostic tool used to detect conditions such as pregnancy, urinary tract infections, metabolic disorders, cancer, and kidney disease. However, contaminated or... Read more
AI-Powered 3D Scanning System Speeds Cancer Screening
Cytology remains a cornerstone of cancer detection, requiring specialists to examine bodily fluids and cells under a microscope. This labor-intensive process involves inspecting up to one million cells... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more










 (3) (1).png)