Cervical Cancer Screening Test Receives Approval for the European Sector
|
By LabMedica International staff writers Posted on 09 Sep 2014 |
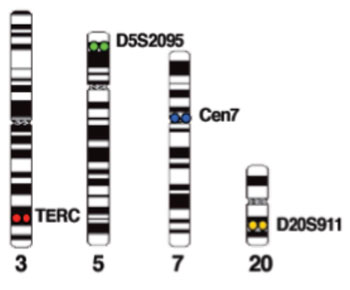
Image: Example of chromosomes stained with the FHACT combination probe: 3q26 (TERC) (red), 5p15 (D5S2095) (green), 20q13 (D20S911) (gold), and CEP7 (aqua) (Photo courtesy of Cancer Genetics Inc.).
A screening test that detects the clonal chromosomal aberrations (gains or amplifications) commonly found in cervical cancer specimens has received CE-marking that allows it to be distributed as a diagnostic test in the 28 countries making up the European Economic Area.
The Cancer Genetics Inc. (Rutherford, NJ, USA) FISH (fluorescence in situ hybridization)-based HPV-Associated Cancer Test (FHACT) utilizes the only four color FISH probe that can be used for cervical cancer screening as additional triage before referral for colposcopy.
The FISH analysis is performed on nuclei of cells derived from a liquid pap specimen using the FHACT combination probe manufactured by CGI Italia (Milan, Italy), a wholly owned subsidiary of Cancer Genetics Inc. The probe comprises 3q26 (TERC) (red), 5p15 (D5S2095) (green), 20q13 (D20S911) (gold), and CEP7 (aqua).
The FISH probe has been optimized to identify copy number gains of 3q26, 5p15, chromosome 7, and 20q13. In normal diploid interphase and metaphase cells, FHACT generates two red, two green, two blue, and two gold signals corresponding to the normal chromosomes 3, 5, 7 and 20 respectively. In cells with gain or amplification of 3q26, 5p15, 20q13 and/or chromosome 7, the number of red, green, gold, and/or blue signals will be greater than two each.
The FHACT test identifies irreversible genomic changes associated with progression to cancer. In addition to identifying those women most at risk for cervical disease, it can help reduce unnecessary, invasive testing and over-treatment in women who do not show the genomic abnormalities associated with disease progression.
"We are committed to the highest level of quality in the development and manufacture of our DNA-FISH probes and tests. Our probes are manufactured to meet or exceed all established quality and performance specifications, and comply with relevant safety and regulatory requirements in order to qualify them for CE marking," said Panna Sharma, CEO of Cancer Genetics, Inc. "Cervical cancer screening is evolving, but there remains a need for more definitive and genomically informed diagnosis. Our proprietary cervical cancer test, FHACT, was designed in response to this need. Having it affixed with the CE-mark will allow us to expand our marketing and sales in Europe, and allow for greater adoption of the test globally."
The FHACT method is protected by several US and international patents.
Related Links:
Cancer Genetics Inc.
CGI Italia
The Cancer Genetics Inc. (Rutherford, NJ, USA) FISH (fluorescence in situ hybridization)-based HPV-Associated Cancer Test (FHACT) utilizes the only four color FISH probe that can be used for cervical cancer screening as additional triage before referral for colposcopy.
The FISH analysis is performed on nuclei of cells derived from a liquid pap specimen using the FHACT combination probe manufactured by CGI Italia (Milan, Italy), a wholly owned subsidiary of Cancer Genetics Inc. The probe comprises 3q26 (TERC) (red), 5p15 (D5S2095) (green), 20q13 (D20S911) (gold), and CEP7 (aqua).
The FISH probe has been optimized to identify copy number gains of 3q26, 5p15, chromosome 7, and 20q13. In normal diploid interphase and metaphase cells, FHACT generates two red, two green, two blue, and two gold signals corresponding to the normal chromosomes 3, 5, 7 and 20 respectively. In cells with gain or amplification of 3q26, 5p15, 20q13 and/or chromosome 7, the number of red, green, gold, and/or blue signals will be greater than two each.
The FHACT test identifies irreversible genomic changes associated with progression to cancer. In addition to identifying those women most at risk for cervical disease, it can help reduce unnecessary, invasive testing and over-treatment in women who do not show the genomic abnormalities associated with disease progression.
"We are committed to the highest level of quality in the development and manufacture of our DNA-FISH probes and tests. Our probes are manufactured to meet or exceed all established quality and performance specifications, and comply with relevant safety and regulatory requirements in order to qualify them for CE marking," said Panna Sharma, CEO of Cancer Genetics, Inc. "Cervical cancer screening is evolving, but there remains a need for more definitive and genomically informed diagnosis. Our proprietary cervical cancer test, FHACT, was designed in response to this need. Having it affixed with the CE-mark will allow us to expand our marketing and sales in Europe, and allow for greater adoption of the test globally."
The FHACT method is protected by several US and international patents.
Related Links:
Cancer Genetics Inc.
CGI Italia
Latest Pathology News
- Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
- World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
- Novel mcPCR Technology to Transform Testing of Clinical Samples
- Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
- Molecular Imaging to Reduce Need for Melanoma Biopsies
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
- First-Of-Its-Kind Test Identifies Autism Risk at Birth
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreMolecular Diagnostics
view channel
Simple One-Hour Saliva Test Detects Common Cancers
Early detection is critical for improving cancer outcomes, yet many diagnostic tests rely on invasive procedures such as blood draws or biopsies. Researchers are exploring simpler approaches that could... Read more
Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
Chemotherapy is often highly effective for germ cell tumors, but in a subset of patients, the disease does not respond well to standard treatment. For these individuals, doctors may consider high-dose... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read more
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more








 (3) (1).png)











