Phenotypic Test Identifies Antibiotic-Resistant Bacterial Infections
|
By LabMedica International staff writers Posted on 04 Dec 2018 |

Image: The BD Phoenix identification and susceptibility combo panels (Photo courtesy of Becton, Dickinson and Company).
Carbapenemase-producing organisms (CPO) are gram-negative bacteria that are resistant to the carbapenem class of antibiotics, which are commonly used for severe or high-risk bacterial infections. Mortality related to CPO infection is quite high around the world, with reported rates from 22% to 72%.
A CPO detect test, which offers results in less than 36 hours, is expected to replace time-consuming, manual detection that can take up to 96 hours for results. The CPO detection test will be offered as part of the BD Phoenix gram-negative panels, which already use susceptibility testing to expose bacteria from a specific patient's infection to a variety of potential treatments or candidates to gauge how they respond.
The BD Phoenix CPO detect test has gained US Food and Drug Administration clearance. The test may help hospitals contain the spread of antimicrobial resistance (AMR) by shortening the time it takes to detect CPOs, thereby enabling the earlier implementation of infection control procedures and the initiation of appropriate antibiotic therapies designed for treating these infections.
Kenneth Thomson, PhD, clinical professor of Pathology and Laboratory Medicine at the University of Louisville School of Medicine, said, “The BD Phoenix CPO detect test is a completely new type of phenotypic test, and its range of automation and diagnostic capabilities is unmatched by all other currently marketed tests. It represents a significant advance in meeting an important clinical need for rapid detection of CPOs.”
Steve Conly, vice president and general manager of Microbiology for BD Diagnostic Systems, said, “The BD Phoenix CPO detect test gives laboratories an accurate and cost-effective method to rapidly identify CPOs and support patient management. Along with the BD Phoenix M50 instrument, this automated first-to-market, phenotypic test to detect CPOs, using the BD Phoenix system, expands the BD portfolio of solutions for identification and antimicrobial susceptibility testing and is another example of the company's commitment to providing solutions that help addressing the global burden of antimicrobial resistance.”
Related Links:
University of Louisville School of Medicine
A CPO detect test, which offers results in less than 36 hours, is expected to replace time-consuming, manual detection that can take up to 96 hours for results. The CPO detection test will be offered as part of the BD Phoenix gram-negative panels, which already use susceptibility testing to expose bacteria from a specific patient's infection to a variety of potential treatments or candidates to gauge how they respond.
The BD Phoenix CPO detect test has gained US Food and Drug Administration clearance. The test may help hospitals contain the spread of antimicrobial resistance (AMR) by shortening the time it takes to detect CPOs, thereby enabling the earlier implementation of infection control procedures and the initiation of appropriate antibiotic therapies designed for treating these infections.
Kenneth Thomson, PhD, clinical professor of Pathology and Laboratory Medicine at the University of Louisville School of Medicine, said, “The BD Phoenix CPO detect test is a completely new type of phenotypic test, and its range of automation and diagnostic capabilities is unmatched by all other currently marketed tests. It represents a significant advance in meeting an important clinical need for rapid detection of CPOs.”
Steve Conly, vice president and general manager of Microbiology for BD Diagnostic Systems, said, “The BD Phoenix CPO detect test gives laboratories an accurate and cost-effective method to rapidly identify CPOs and support patient management. Along with the BD Phoenix M50 instrument, this automated first-to-market, phenotypic test to detect CPOs, using the BD Phoenix system, expands the BD portfolio of solutions for identification and antimicrobial susceptibility testing and is another example of the company's commitment to providing solutions that help addressing the global burden of antimicrobial resistance.”
Related Links:
University of Louisville School of Medicine
Latest Microbiology News
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
- Rapid Diagnostic Test Matches Gold Standard for Sepsis Detection
- Rapid POC Tuberculosis Test Provides Results Within 15 Minutes
- Rapid Assay Identifies Bloodstream Infection Pathogens Directly from Patient Samples
- Blood-Based Molecular Signatures to Enable Rapid EPTB Diagnosis
- 15-Minute Blood Test Diagnoses Life-Threatening Infections in Children
- High-Throughput Enteric Panels Detect Multiple GI Bacterial Infections from Single Stool Swab Sample
- Fast Noninvasive Bedside Test Uses Sugar Fingerprint to Detect Fungal Infections
- Rapid Sepsis Diagnostic Device to Enable Personalized Critical Care for ICU Patients
Channels
Clinical Chemistry
view channel
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read more
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read moreMolecular Diagnostics
view channel
New Testing Method Predicts Trauma Patient Recovery Days in Advance
Trauma patients with nearly identical injuries often experience very different recoveries, even when treated similarly. Traditional assessments based on injury severity do not always explain why some patients... Read more
Simple Method Predicts Risk of Brain Tumor Recurrence
Meningioma is the most common type of brain tumor, developing in the membranes surrounding the brain rather than in brain tissue itself. Although often classified as benign, these tumors can cause significant... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more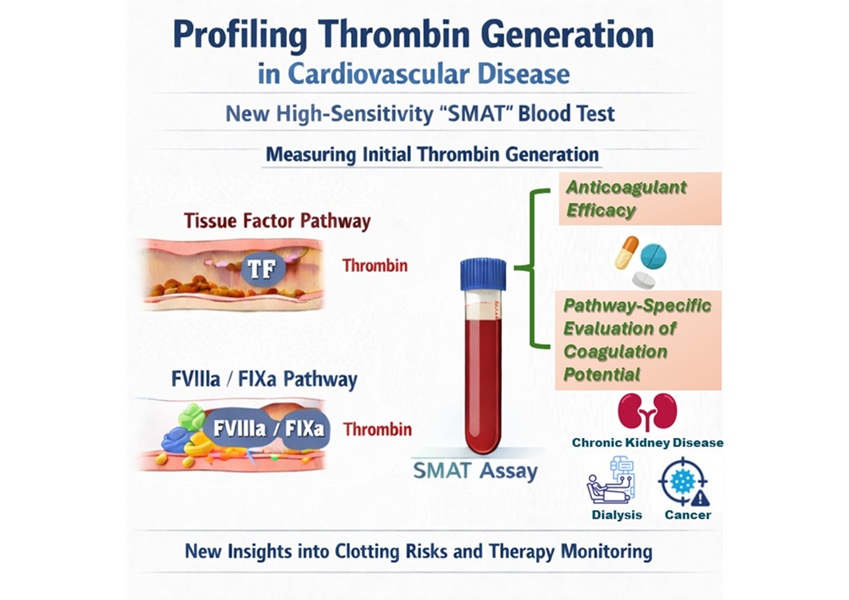
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read morePathology
view channel
AI Tool Helps See How Cells Work Together Inside Diseased Tissue
Microscopes have long been central to diagnosing disease by allowing doctors to examine stained tissue samples. However, modern medical research now generates vast amounts of additional data, including... Read more
AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
Malaria remains one of the world’s deadliest infectious diseases, killing hundreds of thousands each year, mostly in under-resourced regions where laboratory infrastructure is limited. Diagnosis still... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channel
WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
WHX Labs in Dubai (formerly Medlab Middle East), held at Dubai World Trade Centre (DWTC) from 10–13 February, brings together international experts to discuss the factors redefining laboratory leadership,... Read moreNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more