Ebola Test with Portable Reader Authorized for Emergency Use
|
By LabMedica International staff writers Posted on 26 Nov 2018 |
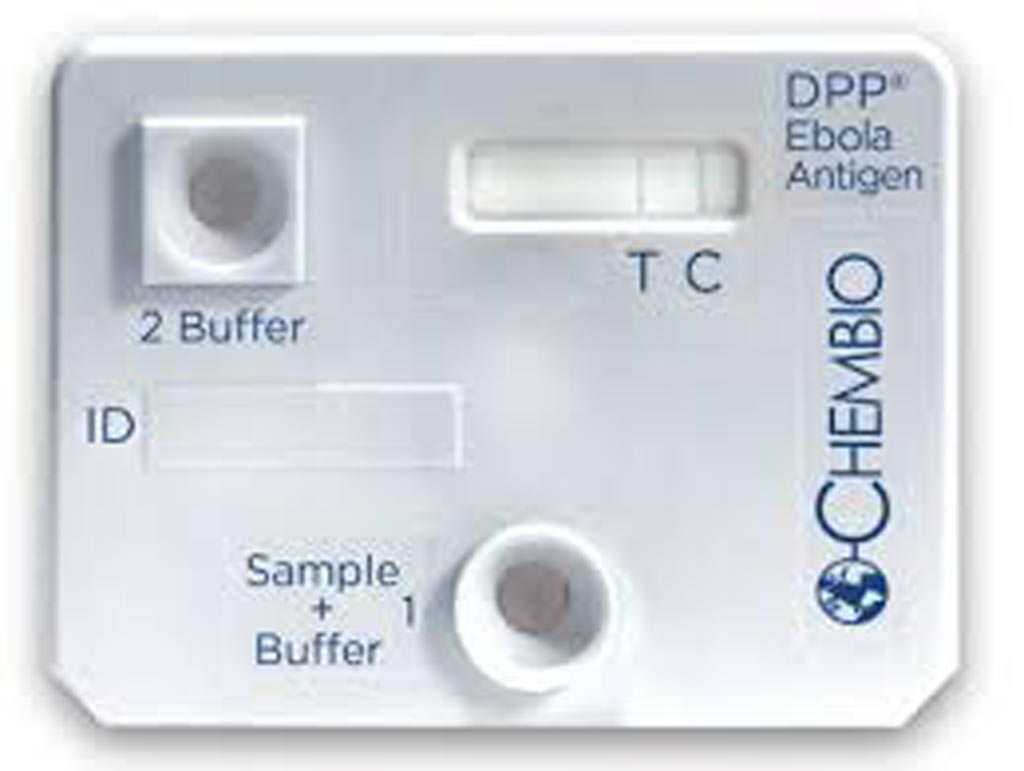
authorization (Photo courtesy of Chembio Diagnostic Systems).
Ebola is a rare but deadly virus that causes fever, body aches, and diarrhea, and sometimes bleeding inside and outside the body. As the virus spreads through the body, it damages the immune system and organs. Ultimately, it causes levels of blood-clotting cells to drop. This leads to severe, uncontrollable bleeding and kills up to 90% of people infected.
The disease is spread from human to human is through contact with blood and/or bodily fluids (urine, saliva, feces, vomit, sweat, breast milk and semen) of infected individuals. This may be through direct contact and/or droplet spread (droplets of infected bodily fluids produced by sneezing, coughing or talking) or via objects (such as needles) and environments that have been contaminated with the virus.
The US Food and Drug Administration (FDA, Silver Springs, MD, USA) issued an emergency use authorization (EUA) for a rapid, single-use test for the detection of Ebola virus (Zaire Ebola virus). This is the second Ebola rapid antigen fingerstick test available under EUA, but the first that uses a portable battery-operated reader, which can help provide clear diagnostic results outside of laboratories and in areas where patients are likely to be treated.
The test, called the DPP Ebola Antigen System (Chembio Diagnostic Systems Inc, Medford, NY, USA) is used with blood specimens, including capillary “fingerstick” whole blood, from individuals with signs and symptoms of Ebola virus disease (EVD) in addition to other risk factors, such as living in an area with large numbers of EVD cases and/or having contact with other individuals exhibiting signs and symptoms of EVD.
The DPP Ebola Antigen System provides rapid diagnostic results with tests that can be performed in locations where a healthcare provider does not have access to authorized Ebola virus nucleic acid tests (PCR testing), which are highly sensitive but can only be performed in certain laboratory settings that are adequately equipped.
The DPP Ebola Antigen System has been authorized for use with capillary “fingerstick” whole blood, ethylenediaminetetraacetic acid (EDTA, an anticoagulant added to whole blood to prevent coagulation) venous whole blood and EDTA plasma. The DPP Ebola Antigen System should only be run in facilities, including treatment centers and public health clinics where patients are likely to be treated, and laboratories that are adequately equipped, trained and capable of such testing.
It is important to note that a negative result from the DPP Ebola Antigen System, especially in patients with signs and symptoms of EVD, should not be used as the sole basis for patient management decisions. The diagnosis of EVD must be made based on multiple factors such as, history, signs, symptoms, exposure likelihood and other laboratory evidence in addition to the detection of Ebola virus.
Related Links:
US Food and Drug Administration
Chembio Diagnostic Systems
The disease is spread from human to human is through contact with blood and/or bodily fluids (urine, saliva, feces, vomit, sweat, breast milk and semen) of infected individuals. This may be through direct contact and/or droplet spread (droplets of infected bodily fluids produced by sneezing, coughing or talking) or via objects (such as needles) and environments that have been contaminated with the virus.
The US Food and Drug Administration (FDA, Silver Springs, MD, USA) issued an emergency use authorization (EUA) for a rapid, single-use test for the detection of Ebola virus (Zaire Ebola virus). This is the second Ebola rapid antigen fingerstick test available under EUA, but the first that uses a portable battery-operated reader, which can help provide clear diagnostic results outside of laboratories and in areas where patients are likely to be treated.
The test, called the DPP Ebola Antigen System (Chembio Diagnostic Systems Inc, Medford, NY, USA) is used with blood specimens, including capillary “fingerstick” whole blood, from individuals with signs and symptoms of Ebola virus disease (EVD) in addition to other risk factors, such as living in an area with large numbers of EVD cases and/or having contact with other individuals exhibiting signs and symptoms of EVD.
The DPP Ebola Antigen System provides rapid diagnostic results with tests that can be performed in locations where a healthcare provider does not have access to authorized Ebola virus nucleic acid tests (PCR testing), which are highly sensitive but can only be performed in certain laboratory settings that are adequately equipped.
The DPP Ebola Antigen System has been authorized for use with capillary “fingerstick” whole blood, ethylenediaminetetraacetic acid (EDTA, an anticoagulant added to whole blood to prevent coagulation) venous whole blood and EDTA plasma. The DPP Ebola Antigen System should only be run in facilities, including treatment centers and public health clinics where patients are likely to be treated, and laboratories that are adequately equipped, trained and capable of such testing.
It is important to note that a negative result from the DPP Ebola Antigen System, especially in patients with signs and symptoms of EVD, should not be used as the sole basis for patient management decisions. The diagnosis of EVD must be made based on multiple factors such as, history, signs, symptoms, exposure likelihood and other laboratory evidence in addition to the detection of Ebola virus.
Related Links:
US Food and Drug Administration
Chembio Diagnostic Systems
Latest Microbiology News
- Rapid Sequencing Could Transform Tuberculosis Care
- Blood-Based Viral Signature Identified in Crohn’s Disease
- Hidden Gut Viruses Linked to Colorectal Cancer Risk
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
- Rapid Diagnostic Test Matches Gold Standard for Sepsis Detection
- Rapid POC Tuberculosis Test Provides Results Within 15 Minutes
- Rapid Assay Identifies Bloodstream Infection Pathogens Directly from Patient Samples
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Simple One-Hour Saliva Test Detects Common Cancers
Early detection is critical for improving cancer outcomes, yet many diagnostic tests rely on invasive procedures such as blood draws or biopsies. Researchers are exploring simpler approaches that could... Read more
Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
Chemotherapy is often highly effective for germ cell tumors, but in a subset of patients, the disease does not respond well to standard treatment. For these individuals, doctors may consider high-dose... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more




















