Genomic Test Detects Lynch Syndrome in Colorectal Cancer
|
By LabMedica International staff writers Posted on 11 Apr 2018 |

Image: UW-OncoPlex is a diagnostic tool that uses genetic sequencing to look for mutations that cause cancer (Photo courtesy of University of Washington).
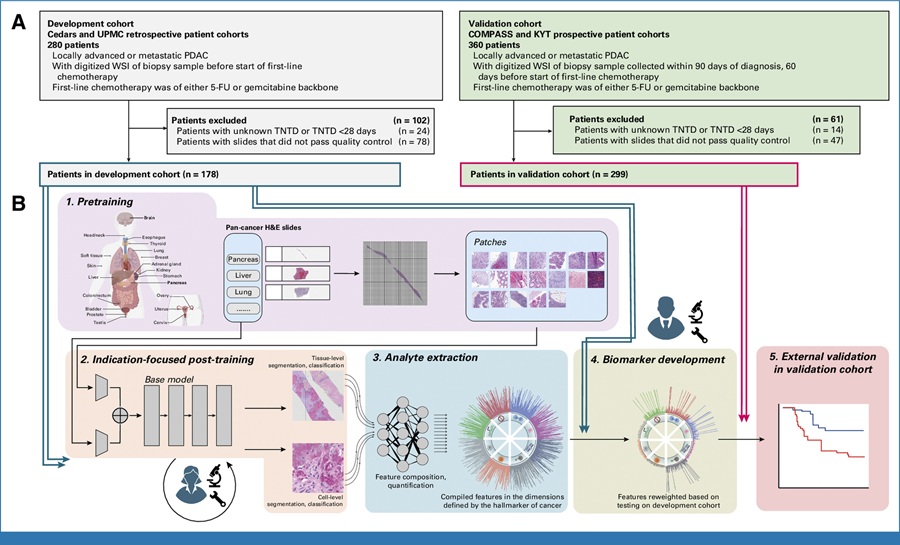
Lynch syndrome (LS) affects approximately 3% of all patients with colorectal cancer (CRC), making it the most common hereditary syndrome that predisposes individuals to develop CRC. Universal tumor screening for LS in CRC is recommended and involves up to six sequential tests.
Somatic gene testing is performed on stage IV CRCs for treatment determination. The diagnostic workup for patients with CRC could be simplified and improved using a single up-front tumor next-generation sequencing test if it has higher sensitivity and specificity than the current screening protocol.
Scientists at Ohio State University (Columbus, OH, USA) and their colleagues analyzed tumor samples from 419 CRC patients who participated in the Ohio Colorectal Cancer Prevention Initiative (OCCPI), a statewide study to screen newly diagnosed CRC patients and their biological relatives for Lynch syndrome. All OCCPI study participants had their tumor samples analyzed using the traditional multi-test genetic testing approach and the single, upfront genomic tumor-sequencing test approach in which a single tumor sample was analyzed for multiple mutations simultaneously. A total of 197 of the 419 prospective cases underwent germline genetic testing.
Tumor sequencing was performed using University of Washington (UW)-OncoPlex in the Clinical Laboratory Improvement Amendments–certified laboratory setting (Seattle, WA, USA). UW-OncoPlex is a clinically validated targeted next-generation sequencing (NGS) deep sequencing panel that sequences to 500 × mean depth all exons, introns, and flanking regions of MLH1, MSH2, and MSH6, and all exons of PMS2 in addition to assessing BRAF, KRAS, NRAS, and MSI status among other genes.
The team compared the results from the two screening methods and found that the upfront tumor-sequencing approach was more sensitive and more specific for detecting Lynch syndrome than the old, multiple-test model. Tumor sequencing resulted in a 10% improvement in Lynch syndrome detection rates while also providing important information about treatment options for the patients.
Heather Hampel, MS, CGC, the principal investigator and corresponding author of the study, said, “Testing methods of the past would just point to a suspicion of Lynch syndrome, but they could not confirm the diagnosis without multiple additional tests, which slows down the diagnostic process and adds costs. This new approach points to the exact mutation patients were born with and does so through a single test. The mutation will need to be confirmed using a blood test but this requires a single mutation test, which is less expensive than multi-gene panel testing. The previous method could sometimes require patients to get up to five individual tests before knowing if they had Lynch syndrome.” The study was published on March 29, 2018, in the journal JAMA Oncology.
Related Links:
Ohio State University
University of Washington
Somatic gene testing is performed on stage IV CRCs for treatment determination. The diagnostic workup for patients with CRC could be simplified and improved using a single up-front tumor next-generation sequencing test if it has higher sensitivity and specificity than the current screening protocol.
Scientists at Ohio State University (Columbus, OH, USA) and their colleagues analyzed tumor samples from 419 CRC patients who participated in the Ohio Colorectal Cancer Prevention Initiative (OCCPI), a statewide study to screen newly diagnosed CRC patients and their biological relatives for Lynch syndrome. All OCCPI study participants had their tumor samples analyzed using the traditional multi-test genetic testing approach and the single, upfront genomic tumor-sequencing test approach in which a single tumor sample was analyzed for multiple mutations simultaneously. A total of 197 of the 419 prospective cases underwent germline genetic testing.
Tumor sequencing was performed using University of Washington (UW)-OncoPlex in the Clinical Laboratory Improvement Amendments–certified laboratory setting (Seattle, WA, USA). UW-OncoPlex is a clinically validated targeted next-generation sequencing (NGS) deep sequencing panel that sequences to 500 × mean depth all exons, introns, and flanking regions of MLH1, MSH2, and MSH6, and all exons of PMS2 in addition to assessing BRAF, KRAS, NRAS, and MSI status among other genes.
The team compared the results from the two screening methods and found that the upfront tumor-sequencing approach was more sensitive and more specific for detecting Lynch syndrome than the old, multiple-test model. Tumor sequencing resulted in a 10% improvement in Lynch syndrome detection rates while also providing important information about treatment options for the patients.
Heather Hampel, MS, CGC, the principal investigator and corresponding author of the study, said, “Testing methods of the past would just point to a suspicion of Lynch syndrome, but they could not confirm the diagnosis without multiple additional tests, which slows down the diagnostic process and adds costs. This new approach points to the exact mutation patients were born with and does so through a single test. The mutation will need to be confirmed using a blood test but this requires a single mutation test, which is less expensive than multi-gene panel testing. The previous method could sometimes require patients to get up to five individual tests before knowing if they had Lynch syndrome.” The study was published on March 29, 2018, in the journal JAMA Oncology.
Related Links:
Ohio State University
University of Washington
Latest Pathology News
- AI Tool Predicts Chemotherapy Response from Biopsy Slides
- Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
- World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
- Novel mcPCR Technology to Transform Testing of Clinical Samples
- Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
- Molecular Imaging to Reduce Need for Melanoma Biopsies
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read more
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more