Enzyme Signpost Points to Better Bowel Cancer Test
|
By LabMedica International staff writers Posted on 20 Mar 2016 |
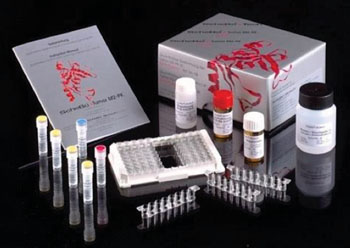
Image: The ScheBo Tumor M2-PK Stool sandwich enzyme-linked immunosorbent assay (Photo courtesy of ScheBo Biotech AG).
A sensitive screening tool for colorectal cancer that detects a special isoenzyme of pyruvate kinase, termed M2-PK, which leaks from the cancerous tissue into the bowel, can then be found in feces.
One alteration consistently found during tumor formation, including gastrointestinal tumors, is the upregulation of glycolytic enzymes. This upregulation takes place at the ribonucleic acid (RNA) and protein level, as well as at the level of enzymatic activities.
The UK Government is currently considering the introduction of a national bowel-screening program. One of the screening tests under consideration is based on the work done several years ago by scientists at the Giessen University Hospital, (Germany) and their colleagues. The team asked patients, given appointments for colonoscopy for various reasons, to provide one stool sample for measuring fecal Tumor M2-PK. Endoscopies were carried out as standard investigations. Histology was obtained from the routine biopsies and/or from surgery. In all, 60 patients with colorectal cancer have been evaluated.
Stool samples of patients with colorectal cancer and patients without pathological findings were tested. Tumor M2-PK was measured with a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) (ScheBo Biotech AG; Giessen, Germany). The ELISA plate is coated with a monoclonal antibody against Tumor M2-PK. Tumor M2-PK from stool samples or standards binds to the antibody. A second monoclonal antibody, which is biotinylated, binds to Tumor M2-PK during the next incubation. Both monoclonal antibodies against Tumor M2-PK specifically react with Tumor M2-PK (dimeric form of M2-PK) and do not cross-react with the other isoforms of pyruvate kinase (type L, R, M1 and tetrameric M2-PK).
There was a highly significant difference between tumor patients and controls. At a cutoff level of 4 UmL-1 the sensitivity was calculated to be 73% and the specificity as 78%. The intra-assay variance was evaluated by 18-fold determination of five samples (5–66 UmL-1), giving an average coefficient of variance (CV) of 7.9% (3.5%–13.6%). The interassay variance was calculated with five samples between 4 and 73 UmL-1, tested on 10 different days. The average CV was 7.3% (3.8%–12.6%).
Robert Souhami, CBE, FMedSci, Director of Policy and Communication for Cancer Research UK, said, “There is currently much interest in this area of investigation. We hope that enzymes such as this one will eventually offer not only useful screening tools, but also an effective method of monitoring bowel cancer patients in remission, so that any return of disease can be quickly detected and acted upon.”
Related Links:
Giessen University Hospital
ScheBo Biotech AG
One alteration consistently found during tumor formation, including gastrointestinal tumors, is the upregulation of glycolytic enzymes. This upregulation takes place at the ribonucleic acid (RNA) and protein level, as well as at the level of enzymatic activities.
The UK Government is currently considering the introduction of a national bowel-screening program. One of the screening tests under consideration is based on the work done several years ago by scientists at the Giessen University Hospital, (Germany) and their colleagues. The team asked patients, given appointments for colonoscopy for various reasons, to provide one stool sample for measuring fecal Tumor M2-PK. Endoscopies were carried out as standard investigations. Histology was obtained from the routine biopsies and/or from surgery. In all, 60 patients with colorectal cancer have been evaluated.
Stool samples of patients with colorectal cancer and patients without pathological findings were tested. Tumor M2-PK was measured with a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) (ScheBo Biotech AG; Giessen, Germany). The ELISA plate is coated with a monoclonal antibody against Tumor M2-PK. Tumor M2-PK from stool samples or standards binds to the antibody. A second monoclonal antibody, which is biotinylated, binds to Tumor M2-PK during the next incubation. Both monoclonal antibodies against Tumor M2-PK specifically react with Tumor M2-PK (dimeric form of M2-PK) and do not cross-react with the other isoforms of pyruvate kinase (type L, R, M1 and tetrameric M2-PK).
There was a highly significant difference between tumor patients and controls. At a cutoff level of 4 UmL-1 the sensitivity was calculated to be 73% and the specificity as 78%. The intra-assay variance was evaluated by 18-fold determination of five samples (5–66 UmL-1), giving an average coefficient of variance (CV) of 7.9% (3.5%–13.6%). The interassay variance was calculated with five samples between 4 and 73 UmL-1, tested on 10 different days. The average CV was 7.3% (3.8%–12.6%).
Robert Souhami, CBE, FMedSci, Director of Policy and Communication for Cancer Research UK, said, “There is currently much interest in this area of investigation. We hope that enzymes such as this one will eventually offer not only useful screening tools, but also an effective method of monitoring bowel cancer patients in remission, so that any return of disease can be quickly detected and acted upon.”
Related Links:
Giessen University Hospital
ScheBo Biotech AG
Latest Immunology News
- Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
- Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
- New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
- Routine Blood Test Can Predict Who Benefits Most from CAR T-Cell Therapy
- New Test Distinguishes Vaccine-Induced False Positives from Active HIV Infection
- Gene Signature Test Predicts Response to Key Breast Cancer Treatment
- Chip Captures Cancer Cells from Blood to Help Select Right Breast Cancer Treatment
- Blood-Based Liquid Biopsy Model Analyzes Immunotherapy Effectiveness
- Signature Genes Predict T-Cell Expansion in Cancer Immunotherapy
- Molecular Microscope Diagnostic System Assesses Lung Transplant Rejection
- Blood Test Tracks Treatment Resistance in High-Grade Serous Ovarian Cancer
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Blood Test Could Spot Common Post-Surgery Condition Early
Heterotopic ossification (HO), the abnormal formation of bone in soft tissue, is a common complication following hip replacement surgery. The condition affects nearly one in three patients and can lead... Read more
New Blood Test Can Help Predict Testicular Cancer Recurrence
Stage 1 testicular germ cell tumor is typically treated with surgery followed by active surveillance. Although most patients experience strong long-term outcomes, about one in four will see their cancer... Read more
New Test Detects Alzheimer’s by Analyzing Altered Protein Shapes in Blood
Alzheimer’s disease begins developing years before memory loss or other symptoms become visible. Misfolded proteins gradually accumulate in the brain, disrupting normal cellular processes.... Read more
New Diagnostic Markers for Multiple Sclerosis Discovered in Cerebrospinal Fluid
Multiple sclerosis (MS) affects nearly three million people worldwide and can cause symptoms such as numbness, visual disturbances, fatigue, and neurological disability. Diagnosing the disease can be challenging... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read more
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more








 (3) (1).png)









