Enzyme Signpost Points to Better Bowel Cancer Test
|
By LabMedica International staff writers Posted on 20 Mar 2016 |
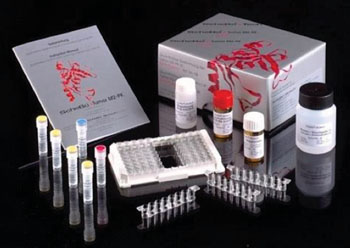
Image: The ScheBo Tumor M2-PK Stool sandwich enzyme-linked immunosorbent assay (Photo courtesy of ScheBo Biotech AG).
A sensitive screening tool for colorectal cancer that detects a special isoenzyme of pyruvate kinase, termed M2-PK, which leaks from the cancerous tissue into the bowel, can then be found in feces.
One alteration consistently found during tumor formation, including gastrointestinal tumors, is the upregulation of glycolytic enzymes. This upregulation takes place at the ribonucleic acid (RNA) and protein level, as well as at the level of enzymatic activities.
The UK Government is currently considering the introduction of a national bowel-screening program. One of the screening tests under consideration is based on the work done several years ago by scientists at the Giessen University Hospital, (Germany) and their colleagues. The team asked patients, given appointments for colonoscopy for various reasons, to provide one stool sample for measuring fecal Tumor M2-PK. Endoscopies were carried out as standard investigations. Histology was obtained from the routine biopsies and/or from surgery. In all, 60 patients with colorectal cancer have been evaluated.
Stool samples of patients with colorectal cancer and patients without pathological findings were tested. Tumor M2-PK was measured with a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) (ScheBo Biotech AG; Giessen, Germany). The ELISA plate is coated with a monoclonal antibody against Tumor M2-PK. Tumor M2-PK from stool samples or standards binds to the antibody. A second monoclonal antibody, which is biotinylated, binds to Tumor M2-PK during the next incubation. Both monoclonal antibodies against Tumor M2-PK specifically react with Tumor M2-PK (dimeric form of M2-PK) and do not cross-react with the other isoforms of pyruvate kinase (type L, R, M1 and tetrameric M2-PK).
There was a highly significant difference between tumor patients and controls. At a cutoff level of 4 UmL-1 the sensitivity was calculated to be 73% and the specificity as 78%. The intra-assay variance was evaluated by 18-fold determination of five samples (5–66 UmL-1), giving an average coefficient of variance (CV) of 7.9% (3.5%–13.6%). The interassay variance was calculated with five samples between 4 and 73 UmL-1, tested on 10 different days. The average CV was 7.3% (3.8%–12.6%).
Robert Souhami, CBE, FMedSci, Director of Policy and Communication for Cancer Research UK, said, “There is currently much interest in this area of investigation. We hope that enzymes such as this one will eventually offer not only useful screening tools, but also an effective method of monitoring bowel cancer patients in remission, so that any return of disease can be quickly detected and acted upon.”
Related Links:
Giessen University Hospital
ScheBo Biotech AG
One alteration consistently found during tumor formation, including gastrointestinal tumors, is the upregulation of glycolytic enzymes. This upregulation takes place at the ribonucleic acid (RNA) and protein level, as well as at the level of enzymatic activities.
The UK Government is currently considering the introduction of a national bowel-screening program. One of the screening tests under consideration is based on the work done several years ago by scientists at the Giessen University Hospital, (Germany) and their colleagues. The team asked patients, given appointments for colonoscopy for various reasons, to provide one stool sample for measuring fecal Tumor M2-PK. Endoscopies were carried out as standard investigations. Histology was obtained from the routine biopsies and/or from surgery. In all, 60 patients with colorectal cancer have been evaluated.
Stool samples of patients with colorectal cancer and patients without pathological findings were tested. Tumor M2-PK was measured with a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) (ScheBo Biotech AG; Giessen, Germany). The ELISA plate is coated with a monoclonal antibody against Tumor M2-PK. Tumor M2-PK from stool samples or standards binds to the antibody. A second monoclonal antibody, which is biotinylated, binds to Tumor M2-PK during the next incubation. Both monoclonal antibodies against Tumor M2-PK specifically react with Tumor M2-PK (dimeric form of M2-PK) and do not cross-react with the other isoforms of pyruvate kinase (type L, R, M1 and tetrameric M2-PK).
There was a highly significant difference between tumor patients and controls. At a cutoff level of 4 UmL-1 the sensitivity was calculated to be 73% and the specificity as 78%. The intra-assay variance was evaluated by 18-fold determination of five samples (5–66 UmL-1), giving an average coefficient of variance (CV) of 7.9% (3.5%–13.6%). The interassay variance was calculated with five samples between 4 and 73 UmL-1, tested on 10 different days. The average CV was 7.3% (3.8%–12.6%).
Robert Souhami, CBE, FMedSci, Director of Policy and Communication for Cancer Research UK, said, “There is currently much interest in this area of investigation. We hope that enzymes such as this one will eventually offer not only useful screening tools, but also an effective method of monitoring bowel cancer patients in remission, so that any return of disease can be quickly detected and acted upon.”
Related Links:
Giessen University Hospital
ScheBo Biotech AG
Latest Immunology News
- Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
- Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
- New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
- Blood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
- Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
- Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
- Blood Test Could Identify Colon Cancer Patients to Benefit from NSAIDs
- Blood Test Could Detect Adverse Immunotherapy Effects
- Routine Blood Test Can Predict Who Benefits Most from CAR T-Cell Therapy
- New Test Distinguishes Vaccine-Induced False Positives from Active HIV Infection
- Gene Signature Test Predicts Response to Key Breast Cancer Treatment
- Chip Captures Cancer Cells from Blood to Help Select Right Breast Cancer Treatment
- Blood-Based Liquid Biopsy Model Analyzes Immunotherapy Effectiveness
- Signature Genes Predict T-Cell Expansion in Cancer Immunotherapy
- Molecular Microscope Diagnostic System Assesses Lung Transplant Rejection
- Blood Test Tracks Treatment Resistance in High-Grade Serous Ovarian Cancer
Channels
Clinical Chemistry
view channel
Blood Test Tracks Transplant Health Using Donor DNA
Organ transplantation offers life-saving treatment for patients with end-stage disease, but complications such as rejection remain a constant risk. Monitoring transplanted organs typically relies on invasive... Read more
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreMolecular Diagnostics
view channel
DNA Aptamers Offer New Tool for Easy Alzheimer's Blood Test
Alzheimer’s disease is the most common cause of dementia and is marked by progressive loss of nerve cells that begins many years before symptoms become noticeable. Detecting early signs of neurodegeneration... Read more
Jumping "DNA Parasites” Linked to Early Tumor Development
Cancer genomes accumulate complex structural variants that can be difficult to resolve with standard short-read sequencing, obscuring clinically relevant drivers of disease. Transposable elements, particularly... Read more
AI-Based Liquid Biopsy Detects Liver Fibrosis, Cirrhosis and Chronic Disease Signals
Liver fibrosis and cirrhosis often develop silently for years before symptoms appear, making early diagnosis difficult. Detecting these conditions earlier could allow treatment before irreversible damage... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
Study Highlights Accuracy Gaps in Consumer Gut Microbiome Kits
Direct-to-consumer gut microbiome kits promise personalized insights by profiling fecal bacteria and generating health readouts, but their analytical accuracy remains uncertain. A new study shows that... Read more
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Agilent Technologies Acquires Pathology Diagnostics Company Biocare Medical
Agilent Technologies (Santa Clara, CA, USA) has entered into a definitive agreement to acquire Biocare Medical (Pacheco, CA, USA), expanding its pathology portfolio through the addition of highly complementary... Read more
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more


















