Noninvasive Test Helps Identify Cause of Specific Kidney Disease
|
By LabMedica International staff writers Posted on 02 Jul 2014 |
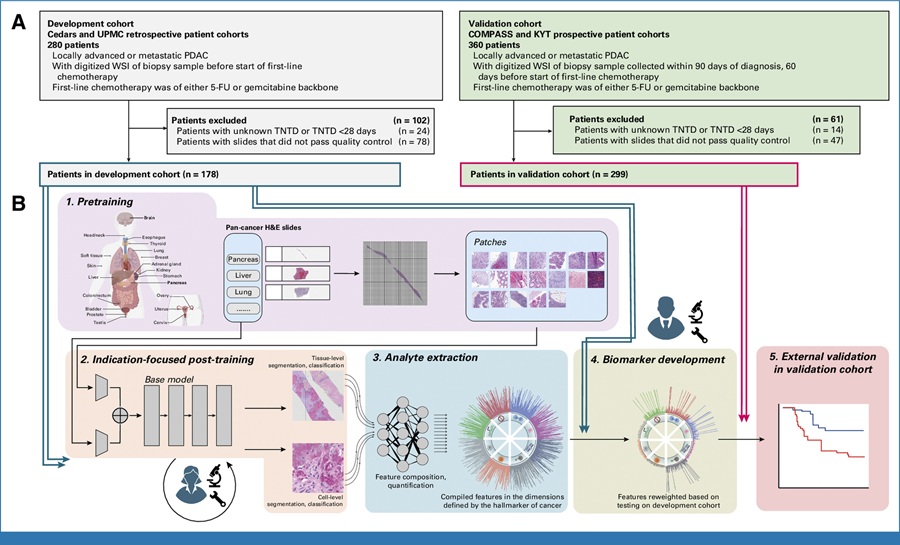
Image: Histopathology of a kidney showing membranous glomerulonephritis (Photo courtesy of University of Utah).
The first test that can help determine a specific type of kidney disease, called membranous glomerulonephritis (MGN) has been authorized for marketing.
Membranous glomerulonephritis (MGN) is a chronic kidney disease that causes damage to the glomeruli, which are the cluster of tiny tufts of capillary blood vessels in the kidney that filter the blood and begin the process to remove waste and excess fluid from the blood.
The US Food and Drug Administration (Silver Springs, MD, USA) reviewed a clinical study of 560 blood samples of which 275 samples were obtained from patients with presumed primary MGN (pMGN), while 285 samples were obtained from patients diagnosed with other kidney diseases including secondary MGN (sMGM) and autoimmune diseases, not including pMGN, that can damage the kidney, like lupus erythematosus.
The EUROIMMUN Anti- PLA2R IFA blood test (Euroimmun US Inc.; Morris Plains, NJ, USA) detects if a patient has an antibody, a protein molecule the body’s immune system produces, that is specific to pMGN. The test was able to detect pMGN in 77% of the presumed pMGN samples, and gave a false positive result in less than 1% of the other disease samples. Notably, the test was helpful in distinguishing between pMGN from sMGN in most of the patients. The test should not be used alone to diagnose pMGN. Additional information, including patient symptoms and other laboratory tests, should always be considered when making a diagnosis of pMGN. A biopsy of the kidney is needed to confirm the diagnosis of pMGN. A negative result from the test does not rule out a diagnosis of pMGN.
Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostics at the FDA, said, “Treatment of MGN depends on the underlying cause of the disease. This test can help patients get a timely diagnosis for their MGN and aid with earlier treatment.” The FDA reviewed the EUROIMMUN Anti- PLA2R IFA blood test through its de novo classification process, a regulatory pathway for some novel low- to moderate-risk medical devices that are first-of-a-kind. The test should not be used to monitor the stage of disease or the response to treatment.
Symptoms of MGN include swelling, high cholesterol, high blood pressure and increased predisposition to blood clots. Over time, usually 10 to 20 years, some people with MGN proceed to kidney failure and require a kidney transplant. MGN affects mostly adult, Caucasian men. About 85% of MGN cases are caused by the body’s immune system mistakenly attacking healthy kidney tissue, which is one of the leading causes of kidney disease in adults.
Related Links:
US Food and Drug Administration
Euroimmun US Inc.
Membranous glomerulonephritis (MGN) is a chronic kidney disease that causes damage to the glomeruli, which are the cluster of tiny tufts of capillary blood vessels in the kidney that filter the blood and begin the process to remove waste and excess fluid from the blood.
The US Food and Drug Administration (Silver Springs, MD, USA) reviewed a clinical study of 560 blood samples of which 275 samples were obtained from patients with presumed primary MGN (pMGN), while 285 samples were obtained from patients diagnosed with other kidney diseases including secondary MGN (sMGM) and autoimmune diseases, not including pMGN, that can damage the kidney, like lupus erythematosus.
The EUROIMMUN Anti- PLA2R IFA blood test (Euroimmun US Inc.; Morris Plains, NJ, USA) detects if a patient has an antibody, a protein molecule the body’s immune system produces, that is specific to pMGN. The test was able to detect pMGN in 77% of the presumed pMGN samples, and gave a false positive result in less than 1% of the other disease samples. Notably, the test was helpful in distinguishing between pMGN from sMGN in most of the patients. The test should not be used alone to diagnose pMGN. Additional information, including patient symptoms and other laboratory tests, should always be considered when making a diagnosis of pMGN. A biopsy of the kidney is needed to confirm the diagnosis of pMGN. A negative result from the test does not rule out a diagnosis of pMGN.
Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostics at the FDA, said, “Treatment of MGN depends on the underlying cause of the disease. This test can help patients get a timely diagnosis for their MGN and aid with earlier treatment.” The FDA reviewed the EUROIMMUN Anti- PLA2R IFA blood test through its de novo classification process, a regulatory pathway for some novel low- to moderate-risk medical devices that are first-of-a-kind. The test should not be used to monitor the stage of disease or the response to treatment.
Symptoms of MGN include swelling, high cholesterol, high blood pressure and increased predisposition to blood clots. Over time, usually 10 to 20 years, some people with MGN proceed to kidney failure and require a kidney transplant. MGN affects mostly adult, Caucasian men. About 85% of MGN cases are caused by the body’s immune system mistakenly attacking healthy kidney tissue, which is one of the leading causes of kidney disease in adults.
Related Links:
US Food and Drug Administration
Euroimmun US Inc.
Latest Pathology News
- AI Tool Predicts Chemotherapy Response from Biopsy Slides
- Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
- World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
- Novel mcPCR Technology to Transform Testing of Clinical Samples
- Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
- Molecular Imaging to Reduce Need for Melanoma Biopsies
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreMolecular Diagnostics
view channel
AI-Powered Blood Test Detects Early Pancreatic Cancer with More Than 90% Accuracy
Pancreatic cancer is one of the most lethal cancers, often referred to as the “King of Cancers” because symptoms usually appear only at advanced stages. As a result, most patients are diagnosed late, and... Read more
AI-Powered Blood Test Flags Relapse Risk Earlier After Transplant
Relapse after allogeneic hematopoietic cell transplant is a major cause of mortality in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), and standard monitoring can miss early warning signals.... Read more
World’s First Portable POC Test Simultaneously Detects Four Common STIs in One Hour
Sexually transmitted infections (STIs) often present with similar symptoms, making accurate diagnosis challenging without laboratory testing. Delays in identifying the exact infection can lead to inappropriate... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreMicrobiology
view channel
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read more
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Agilent Technologies Acquires Pathology Diagnostics Company Biocare Medical
Agilent Technologies (Santa Clara, CA, USA) has entered into a definitive agreement to acquire Biocare Medical (Pacheco, CA, USA), expanding its pathology portfolio through the addition of highly complementary... Read more
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more