Circulating Trophoblast-Based Noninvasive Prenatal Testing Are Feasible
|
By LabMedica International staff writers Posted on 26 Dec 2019 |

Image: A CyteFinder instrument is an automated six channel fluorescence microscopes that rapidly image slides and employ machine learning to identify rare cells (Photo courtesy of RareCyte)
Circulating cells from the fetus or placenta in a pregnant woman’s blood, primarily trophoblasts and nucleated fetal red blood cells (fnRBCs), have long been appreciated as potential targets for noninvasive prenatal testing (NIPT) and diagnosis.
While cell-free DNA-based NIPT has taken off in recent years, a number of groups have been focusing their efforts on fetal cell-based methods, which would enable the analysis of pure fetal DNA instead of a mixture of maternal and fetal genetic material and possibly yield more accurate results with higher resolution.
Molecular Geneticists at Baylor College of Medicine (Houston TX, USA) and their colleagues collected two sets of blood samples from a total of 95 pregnant women who were recruited during prenatal visits, one set of 42 samples and another of 53 samples. Most came were from singleton pregnancies, and gestational age ranged from 8 weeks to 29 weeks.
To enrich trophoblasts, the scientists used a previously published positive selection protocol that they had since optimized. It involved antibodies against three trophoblast cell-surface antigens, HLA-G, TROP-2, and EpCAM, and magnetic bead selection. This was followed by immunostaining with anti-cytokeratin and CD45 antibodies. The samples were then spread on a slide and scanned using a CyteFinder instrument (RareCyte, Seattle, WA, USA). After manually identifying trophoblast candidates under the microscope, based on their cytokeratin patterns and the absence of CD45, the team picked individual cells using the RareCyte CytePicker.
The team, on average, identified five to seven trophoblasts per blood sample, with only two samples where no such cells could be found. Subsequent sequencing and genotyping showed that 94% to 96% of the cells scored as trophoblasts under the microscope were indeed of fetal origin. In addition, a little over half the samples had at least two high-quality trophoblasts where the sequencing results could be scored for both aneuploidy and small copy number variants.
For a total of 45 samples, the scientists had diagnostic results from amniocentesis or chorionic villus sampling (CVS) available. For 34 of these, they saw concordance with the single circulating trophoblast (SCT) sequencing results, and for eight cases, the diagnostic results were normal but SCT testing failed.
The authors concluded that SCT analysis is potentially a powerful tool for prenatal testing and diagnosis. They are optimistic that the recovery of trophoblasts can be improved. SCT testing has the potential to deliver a diagnostic result instead of being merely a screening test if an adequate number of trophoblast cells can be obtained for every sampled pregnancy. A longer-term goal would be to detect all de novo point mutations in a fetus. The study was published on November 27, 2019 in the American Journal of Human Genetics.
Related Links:
Baylor College of Medicine
RareCyte
While cell-free DNA-based NIPT has taken off in recent years, a number of groups have been focusing their efforts on fetal cell-based methods, which would enable the analysis of pure fetal DNA instead of a mixture of maternal and fetal genetic material and possibly yield more accurate results with higher resolution.
Molecular Geneticists at Baylor College of Medicine (Houston TX, USA) and their colleagues collected two sets of blood samples from a total of 95 pregnant women who were recruited during prenatal visits, one set of 42 samples and another of 53 samples. Most came were from singleton pregnancies, and gestational age ranged from 8 weeks to 29 weeks.
To enrich trophoblasts, the scientists used a previously published positive selection protocol that they had since optimized. It involved antibodies against three trophoblast cell-surface antigens, HLA-G, TROP-2, and EpCAM, and magnetic bead selection. This was followed by immunostaining with anti-cytokeratin and CD45 antibodies. The samples were then spread on a slide and scanned using a CyteFinder instrument (RareCyte, Seattle, WA, USA). After manually identifying trophoblast candidates under the microscope, based on their cytokeratin patterns and the absence of CD45, the team picked individual cells using the RareCyte CytePicker.
The team, on average, identified five to seven trophoblasts per blood sample, with only two samples where no such cells could be found. Subsequent sequencing and genotyping showed that 94% to 96% of the cells scored as trophoblasts under the microscope were indeed of fetal origin. In addition, a little over half the samples had at least two high-quality trophoblasts where the sequencing results could be scored for both aneuploidy and small copy number variants.
For a total of 45 samples, the scientists had diagnostic results from amniocentesis or chorionic villus sampling (CVS) available. For 34 of these, they saw concordance with the single circulating trophoblast (SCT) sequencing results, and for eight cases, the diagnostic results were normal but SCT testing failed.
The authors concluded that SCT analysis is potentially a powerful tool for prenatal testing and diagnosis. They are optimistic that the recovery of trophoblasts can be improved. SCT testing has the potential to deliver a diagnostic result instead of being merely a screening test if an adequate number of trophoblast cells can be obtained for every sampled pregnancy. A longer-term goal would be to detect all de novo point mutations in a fetus. The study was published on November 27, 2019 in the American Journal of Human Genetics.
Related Links:
Baylor College of Medicine
RareCyte
Latest Technology News
- New Diagnostic System Achieves PCR Testing Accuracy
- DNA Biosensor Enables Early Diagnosis of Cervical Cancer
- Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
- Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
- First of Its Kind Technology Detects Glucose in Human Saliva
- Electrochemical Device Identifies People at Higher Risk for Osteoporosis Using Single Blood Drop
- Novel Noninvasive Test Detects Malaria Infection without Blood Sample
- Portable Optofluidic Sensing Devices Could Simultaneously Perform Variety of Medical Tests
- Point-of-Care Software Solution Helps Manage Disparate POCT Scenarios across Patient Testing Locations
- Electronic Biosensor Detects Biomarkers in Whole Blood Samples without Addition of Reagents
- Breakthrough Test Detects Biological Markers Related to Wider Variety of Cancers
- Rapid POC Sensing Kit to Determine Gut Health from Blood Serum and Stool Samples
- Device Converts Smartphone into Fluorescence Microscope for Just USD 50
- Wi-Fi Enabled Handheld Tube Reader Designed for Easy Portability
Channels
Clinical Chemistry
view channel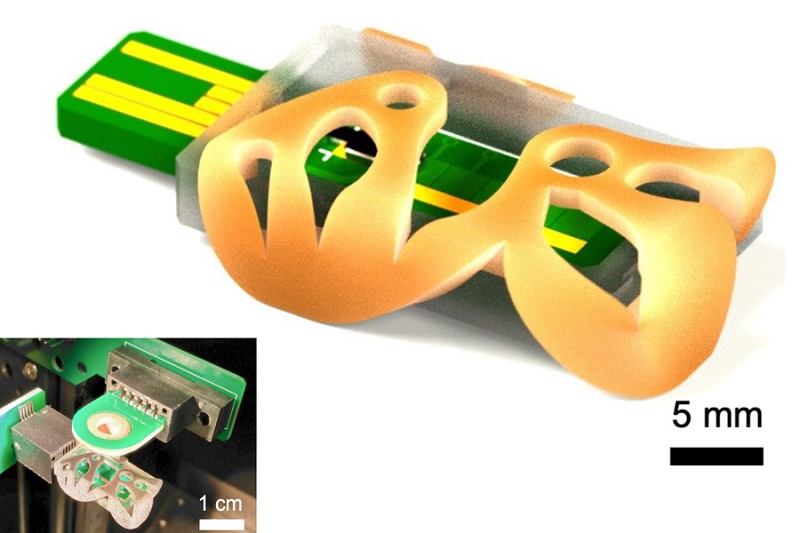
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more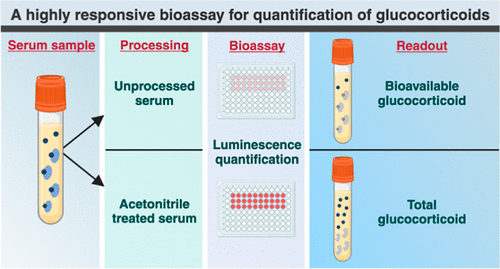
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channelBlood Proteins Could Warn of Cancer Seven Years before Diagnosis
Two studies have identified proteins in the blood that could potentially alert individuals to the presence of cancer more than seven years before the disease is clinically diagnosed. Researchers found... Read moreUltrasound-Aided Blood Testing Detects Cancer Biomarkers from Cells
Ultrasound imaging serves as a noninvasive method to locate and monitor cancerous tumors effectively. However, crucial details about the cancer, such as the specific types of cells and genetic mutations... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more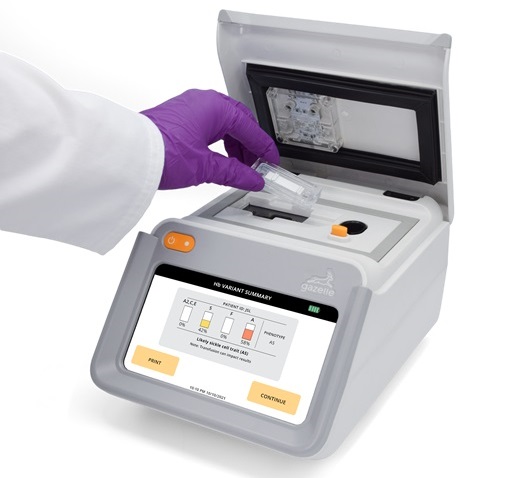
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel.jpg)
AI Predicts Tumor-Killing Cells with High Accuracy
Cellular immunotherapy involves extracting immune cells from a patient's tumor, potentially enhancing their cancer-fighting capabilities through engineering, and then expanding and reintroducing them into the body.... Read more
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channelHyperspectral Dark-Field Microscopy Enables Rapid and Accurate Identification of Cancerous Tissues
Breast cancer remains a major cause of cancer-related mortality among women. Breast-conserving surgery (BCS), also known as lumpectomy, is the removal of the cancerous lump and a small margin of surrounding tissue.... Read more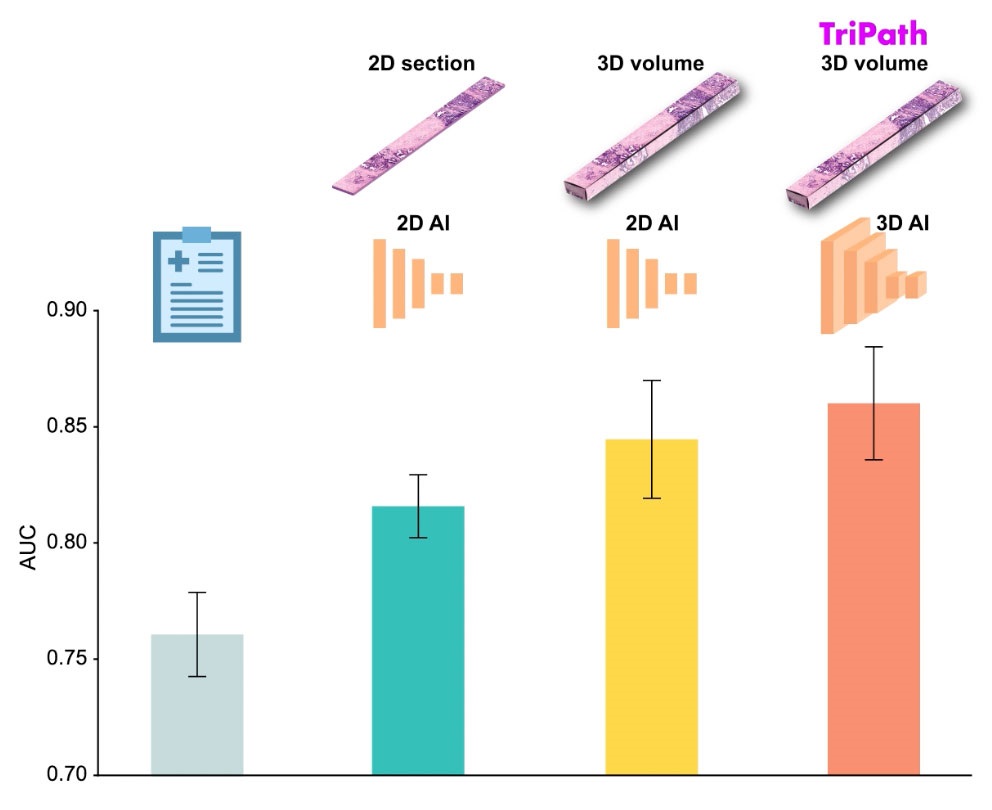
AI Advancements Enable Leap into 3D Pathology
Human tissue is complex, intricate, and naturally three-dimensional. However, the thin two-dimensional tissue slices commonly used by pathologists to diagnose diseases provide only a limited view of the... Read more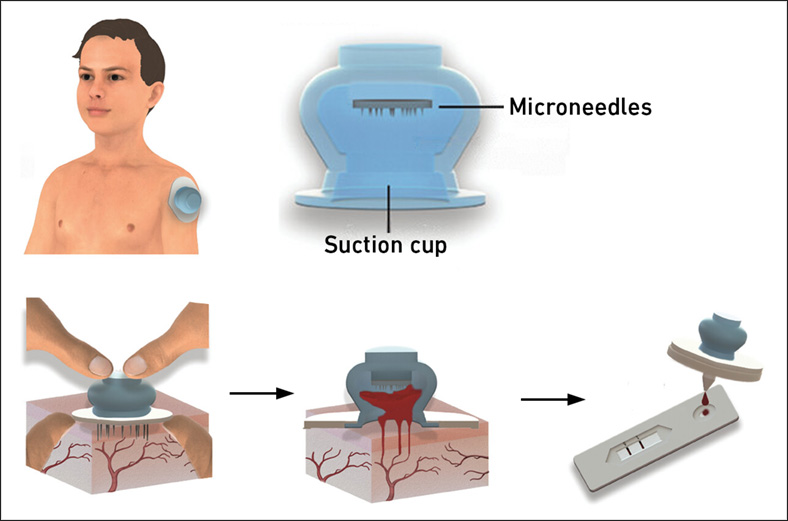
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more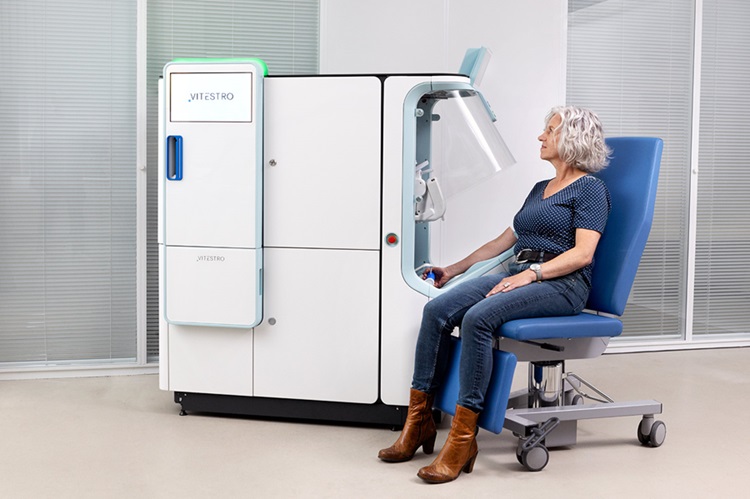
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read moreIndustry
view channel
Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
Unlike severe traumatic brain injury (TBI), mild TBI often does not show clear correlations with abnormalities detected through head computed tomography (CT) scans. Consequently, there is a pressing need... Read more
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)












_1.jpg)
.jpg)
