Blood Test Diagnoses Pancreatic Cancer Disease Earlier
|
By LabMedica International staff writers Posted on 29 Jan 2019 |
Image: Immunocytochemistry using anti- TRA-1-60 antibody (Photo courtesy of Novus Biologicals).
Pancreatic cancer is difficult to diagnose because it often does not have obvious early symptoms; as by the time the disease is found, it typically is quite advanced, complicating treatment and leading to poorer outcomes.
Only 8.5% of people with pancreatic cancer survive past five years, a figure that has risen just slightly since the early 1990s. A new, simple blood test has been developed that, when combined with an existing test, detects nearly 70% of pancreatic cancers with a less than 5% false-positive rate.
Scientists at the Van Andel Research Institute (Grand Rapids, MI, USA) and their colleagues collected blood samples from patients with pancreatic cancer or a benign condition involving the pancreas, and from healthy subjects. The team combined the current biomarker Carbohydrate antigen CA19-9 with a sialylated keratan sulfate proteoglycan sugar called sTRA that was measured by the new test, which is produced by a different subset of pancreatic cancers.
The team developed candidate biomarkers from sTRA and CA19-9 in a training set of 147 plasma samples and used the panels to make case/control calls, based on predetermined thresholds, in a 50-sample validation set and a blinded, 147-sample test set. The team used sandwich immunoassays utilizing antibody array methods with slight modifications. The capture antibodies were CA19-9, anti-MUC5AC, and anti-MUC16. The biotinylated primary antibodies were CA19-9 from MyBioSource or TRA-1-60.
The scientists reported that the two biomarker panels improved upon CA19-9 in the training set, one optimized for specificity, which included CA19-9 and two versions of the sTRA assay, and another optimized for sensitivity, which included two sTRA assays. Both panels achieved statistical improvement over CA19-9 in the validation set, and the specificity-optimized panel achieved statistical improvement in the blinded set: 95% specificity and 54% sensitivity (75% accuracy), compared to 97%/30% (65% accuracy). When the data was unblinded it produced further improvements and revealed independent, complementary contributions from each marker.
The authors concluded that sTRA is a validated serological biomarker of pancreatic ductal adenocarcinoma (PDAC) that yields improved performance over CA19-9. The new panels may enable surveillance for PDAC among people with elevated risk, or improved differential diagnosis among patients with suspected pancreatic cancer. Brian Haab, PhD, a professor and the study’s leading author, said, “We hope that our new test, when used in conjunction with the currently available test, will help doctors catch and treat pancreatic cancer in high-risk individuals before the disease has spread.” The study was published online on January 7, 2019, in the journal Clinical Cancer Research.
Related Links:
Van Andel Research Institute
Only 8.5% of people with pancreatic cancer survive past five years, a figure that has risen just slightly since the early 1990s. A new, simple blood test has been developed that, when combined with an existing test, detects nearly 70% of pancreatic cancers with a less than 5% false-positive rate.
Scientists at the Van Andel Research Institute (Grand Rapids, MI, USA) and their colleagues collected blood samples from patients with pancreatic cancer or a benign condition involving the pancreas, and from healthy subjects. The team combined the current biomarker Carbohydrate antigen CA19-9 with a sialylated keratan sulfate proteoglycan sugar called sTRA that was measured by the new test, which is produced by a different subset of pancreatic cancers.
The team developed candidate biomarkers from sTRA and CA19-9 in a training set of 147 plasma samples and used the panels to make case/control calls, based on predetermined thresholds, in a 50-sample validation set and a blinded, 147-sample test set. The team used sandwich immunoassays utilizing antibody array methods with slight modifications. The capture antibodies were CA19-9, anti-MUC5AC, and anti-MUC16. The biotinylated primary antibodies were CA19-9 from MyBioSource or TRA-1-60.
The scientists reported that the two biomarker panels improved upon CA19-9 in the training set, one optimized for specificity, which included CA19-9 and two versions of the sTRA assay, and another optimized for sensitivity, which included two sTRA assays. Both panels achieved statistical improvement over CA19-9 in the validation set, and the specificity-optimized panel achieved statistical improvement in the blinded set: 95% specificity and 54% sensitivity (75% accuracy), compared to 97%/30% (65% accuracy). When the data was unblinded it produced further improvements and revealed independent, complementary contributions from each marker.
The authors concluded that sTRA is a validated serological biomarker of pancreatic ductal adenocarcinoma (PDAC) that yields improved performance over CA19-9. The new panels may enable surveillance for PDAC among people with elevated risk, or improved differential diagnosis among patients with suspected pancreatic cancer. Brian Haab, PhD, a professor and the study’s leading author, said, “We hope that our new test, when used in conjunction with the currently available test, will help doctors catch and treat pancreatic cancer in high-risk individuals before the disease has spread.” The study was published online on January 7, 2019, in the journal Clinical Cancer Research.
Related Links:
Van Andel Research Institute
Latest Immunology News
- Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
- AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
- Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
- Testing Method Could Help More Patients Receive Right Cancer Treatment
- Groundbreaking Test Monitors Radiation Therapy Toxicity in Cancer Patients
- State-Of-The Art Techniques to Investigate Immune Response in Deadly Strep A Infections
- Novel Immunoassays Enable Early Diagnosis of Antiphospholipid Syndrome
- New Test Could Predict Immunotherapy Success for Broader Range Of Cancers
- Simple Blood Protein Tests Predict CAR T Outcomes for Lymphoma Patients
- Cell Sorter Chip Technology to Pave Way for Immune Profiling at POC
- Chip Monitors Cancer Cells in Blood Samples to Assess Treatment Effectiveness
- Automated Immunohematology Approaches Can Resolve Transplant Incompatibility
- AI Leverages Tumor Genetics to Predict Patient Response to Chemotherapy
- World’s First Portable, Non-Invasive WBC Monitoring Device to Eliminate Need for Blood Draw
- Predictive T-Cell Test Detects Immune Response to Viruses Even Before Antibodies Form
- Single Blood Draw to Detect Immune Cells Present Months before Flu Infection Can Predict Symptoms
Channels
Clinical Chemistry
view channel
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more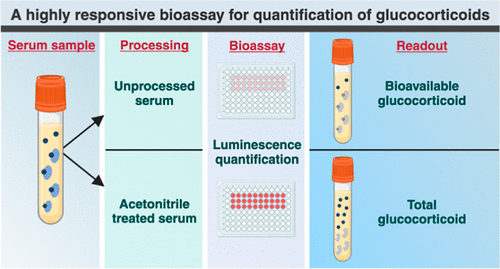
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
Novel Biomarkers to Improve Diagnosis of Renal Cell Carcinoma Subtypes
Renal cell carcinomas (RCCs) are notably diverse, encompassing over 20 distinct subtypes and generally categorized into clear cell and non-clear cell types; around 20% of all RCCs fall into the non-clear... Read more
RNA-Powered Molecular Test to Help Combat Early-Age Onset Colorectal Cancer
Colorectal cancer (CRC) ranks as the second most lethal cancer in the United States. Nevertheless, many Americans eligible for screening do not undergo testing due to limited access or reluctance towards... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channel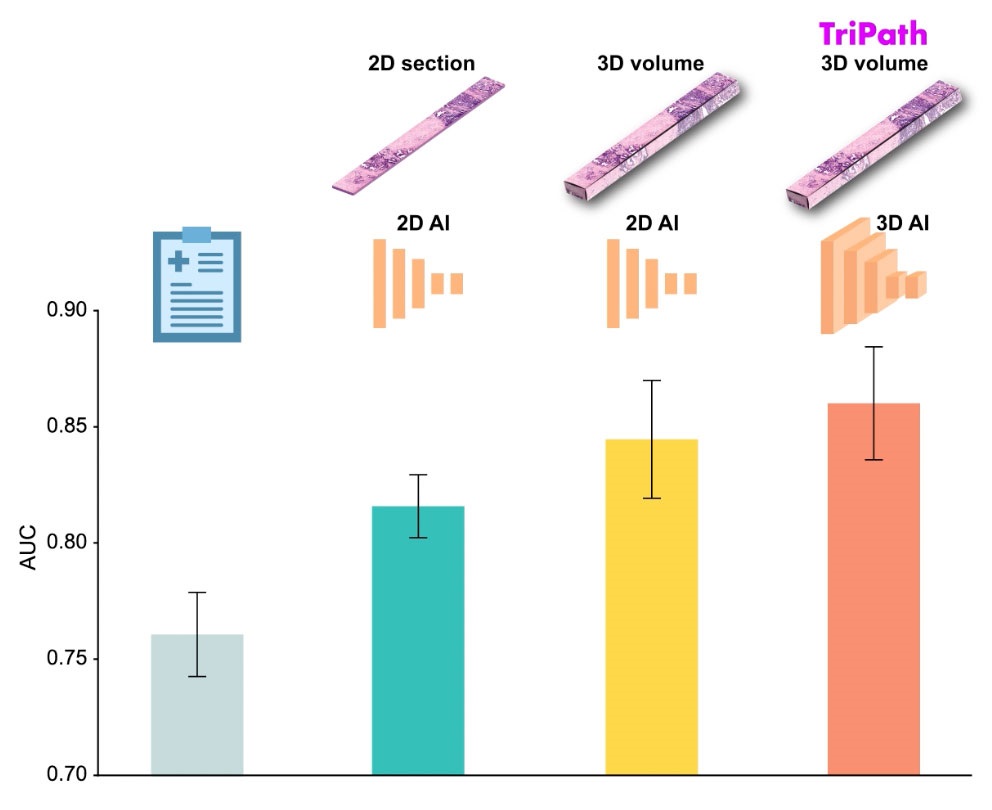
AI Advancements Enable Leap into 3D Pathology
Human tissue is complex, intricate, and naturally three-dimensional. However, the thin two-dimensional tissue slices commonly used by pathologists to diagnose diseases provide only a limited view of the... Read more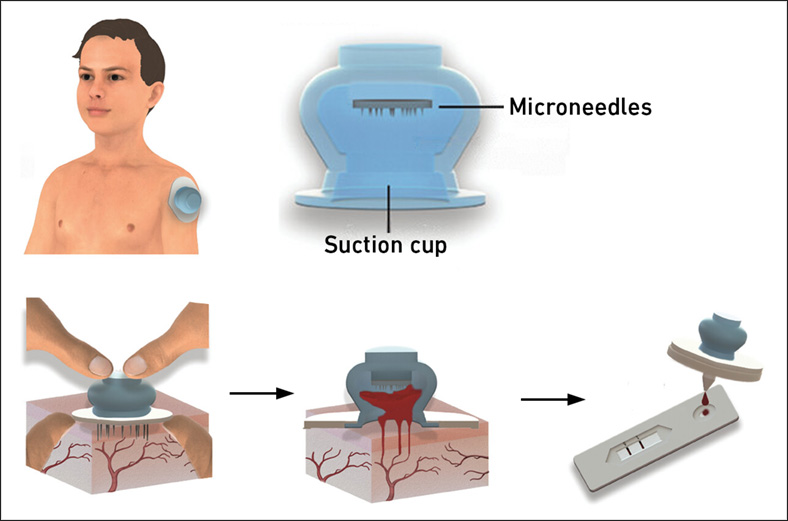
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more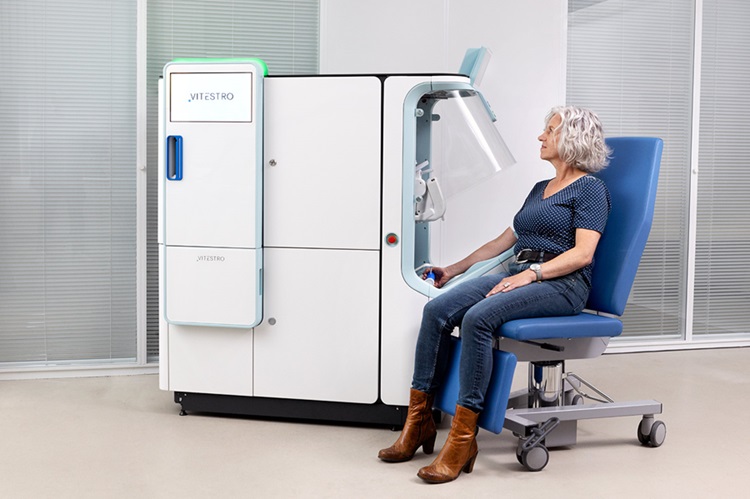
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read more.jpg)
Use of DICOM Images for Pathology Diagnostics Marks Significant Step towards Standardization
Digital pathology is rapidly becoming a key aspect of modern healthcare, transforming the practice of pathology as laboratories worldwide adopt this advanced technology. Digital pathology systems allow... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more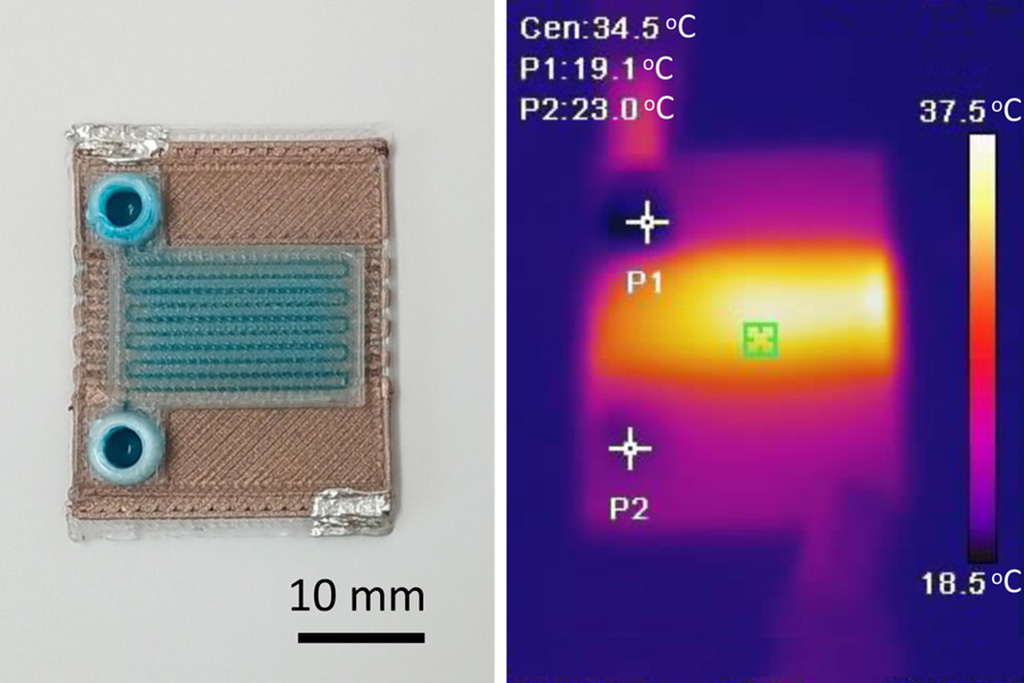
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)
Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
Thermo Fisher Scientific (Waltham, MA USA) has entered into a strategic distribution agreement with Bio-Techne Corporation (Minneapolis, MN, USA), resulting in a significant collaboration between two industry... Read more
ECCMID Congress Name Changes to ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland) has evolved remarkably. The society is now stronger and broader than ever before... Read more