New Automated Chemistry Analyzer Presented at IBMS 2015
|
By LabMedica International staff writers Posted on 11 Oct 2015 |
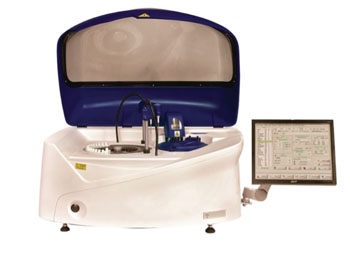
A new clinical chemistry analyzer provides an automated bench-top platform with an integrated chemistry system supported by a broad menu of ready-to-use, bar coded reagents.
EKF Diagnostics (Cardiff, UK) announced the UK launch of its “Altair 240” clinical chemistry analyzer at the Institute of Biomedical Science Congress 2015 (IBMS, September 28-30, 2015; Birmingham, UK). Also highlighted was the latest additions to its range of Stanbio liquid-stable, LiquiColor and Liqui-UV reagents, including an assay for Procalcitonin.
Altair 240 fulfills a need for an automated, cost-effective bench-top platform supported by the reliable Stanbio Chemistry liquid reagent menu for routine and special chemistries. Altair 240 also provides the flexibility to configure open-channel applications for many esoteric assays. Built on 54 years of expertise, the system with LIS bi-directional connectivity is easy-to-use, as either a main or back-up analyzer, and is capable of performing up to 480 tests/hour (double-arm functionality yields up to 480 tests/hour).
Also presented at IBMS 2015 was the Stanbio Chemistry - Procalcitonin (PCT) LiquiColor Assay, a new rapid test enabling the quantitative determination of PCT in serum samples, EDTA, or lithium heparin plasma samples by latex-enhanced immunoturbidimetric methodology. PCT is a marker for bacterial infection and sepsis and has been recognized as an important adjunct marker in the diagnosis of sepsis. The test provides a precise result that correlates well with established methodology, within 10 minutes and requires only 20 µL of sample. The reagents may be used on almost any liquid-based chemistry analyzer with open-channel capability. Reagent kit, calibrator, and control sets are available separately.
Other products showcased at EKF’s IBMS booth included: B-Hydroxybutyrate (B-HB) LiquiColor Assay for ketosis testing; Glycated Serum Protein (GSP) LiquiColor Assay – a 2-3 week indicator of average blood glucose; Quo-Lab and Quo-Test devices for HbA1c measurement; and Hemo Control hemoglobin analyzer.
Related Links:
EKF Diagnostics
EKF Diagnostics (Cardiff, UK) announced the UK launch of its “Altair 240” clinical chemistry analyzer at the Institute of Biomedical Science Congress 2015 (IBMS, September 28-30, 2015; Birmingham, UK). Also highlighted was the latest additions to its range of Stanbio liquid-stable, LiquiColor and Liqui-UV reagents, including an assay for Procalcitonin.
Altair 240 fulfills a need for an automated, cost-effective bench-top platform supported by the reliable Stanbio Chemistry liquid reagent menu for routine and special chemistries. Altair 240 also provides the flexibility to configure open-channel applications for many esoteric assays. Built on 54 years of expertise, the system with LIS bi-directional connectivity is easy-to-use, as either a main or back-up analyzer, and is capable of performing up to 480 tests/hour (double-arm functionality yields up to 480 tests/hour).
Also presented at IBMS 2015 was the Stanbio Chemistry - Procalcitonin (PCT) LiquiColor Assay, a new rapid test enabling the quantitative determination of PCT in serum samples, EDTA, or lithium heparin plasma samples by latex-enhanced immunoturbidimetric methodology. PCT is a marker for bacterial infection and sepsis and has been recognized as an important adjunct marker in the diagnosis of sepsis. The test provides a precise result that correlates well with established methodology, within 10 minutes and requires only 20 µL of sample. The reagents may be used on almost any liquid-based chemistry analyzer with open-channel capability. Reagent kit, calibrator, and control sets are available separately.
Other products showcased at EKF’s IBMS booth included: B-Hydroxybutyrate (B-HB) LiquiColor Assay for ketosis testing; Glycated Serum Protein (GSP) LiquiColor Assay – a 2-3 week indicator of average blood glucose; Quo-Lab and Quo-Test devices for HbA1c measurement; and Hemo Control hemoglobin analyzer.
Related Links:
EKF Diagnostics
Read the full article by registering today, it's FREE! 

Register now for FREE to LabMedica.com and get complete access to news and events that shape the world of Clinical Laboratory Medicine. 
- Free digital version edition of LabMedica International sent by email on regular basis
- Free print version of LabMedica International magazine (available only outside USA and Canada).
- Free and unlimited access to back issues of LabMedica International in digital format
- Free LabMedica International Newsletter sent every week containing the latest news
- Free breaking news sent via email
- Free access to Events Calendar
- Free access to LinkXpress new product services
- REGISTRATION IS FREE AND EASY!
Sign in: Registered website members
Sign in: Registered magazine subscribers
Latest Clinical Chem. News
- 3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
- POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
- Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
- New Blood Testing Method Detects Potent Opioids in Under Three Minutes
- Wireless Hepatitis B Test Kit Completes Screening and Data Collection in One Step
- Pain-Free, Low-Cost, Sensitive, Radiation-Free Device Detects Breast Cancer in Urine
- Spit Test Detects Breast Cancer in Five Seconds
- Electrochemical Sensors with Next-Generation Coating Advances Precision Diagnostics at POC
- First-Of-Its-Kind Handheld Device Accurately Detects Fentanyl in Urine within Seconds
- New Fluorescent Sensor Array Lights up Alzheimer’s-Related Proteins for Earlier Detection
- Automated Mass Spectrometry-Based Clinical Analyzer Could Transform Lab Testing
- Highly Sensitive pH Sensor to Aid Detection of Cancers and Vector-Borne Viruses
- Non-Invasive Sensor Monitors Changes in Saliva Compositions to Rapidly Diagnose Diabetes
- Breakthrough Immunoassays to Aid in Risk Assessment of Preeclampsia
- Urine Test for Monitoring Changes in Kidney Health Markers Can Predict New-Onset Heart Failure
- AACC Releases Comprehensive Diabetes Testing Guidelines
Channels
Molecular Diagnostics
view channel
Advanced Blood Test to Spot Alzheimer's Before Progression to Dementia
Alzheimer’s disease is well known for its slow development over many years, which typically leads to treatment interventions only after the disease has advanced to stages where it may be nearly impossible... Read more
Multi-Omic Noninvasive Urine-Based DNA Test to Improve Bladder Cancer Detection
Hematuria, the presence of blood in urine, is commonly associated with bladder cancer and is its most frequent symptom. Current guidelines recommend referring patients with hematuria to urology for clinical... Read more.jpg)
First of Its Kind NGS Assay for Precise Detection of BCR::ABL1 Fusion Gene to Enable Personalized Leukemia Treatment
The BCR::ABL1 fusion gene plays a key role in the pathogenesis of several blood cancers, particularly chronic myeloid leukemia (CML). This gene results from a chromosomal translocation that causes constitutive... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more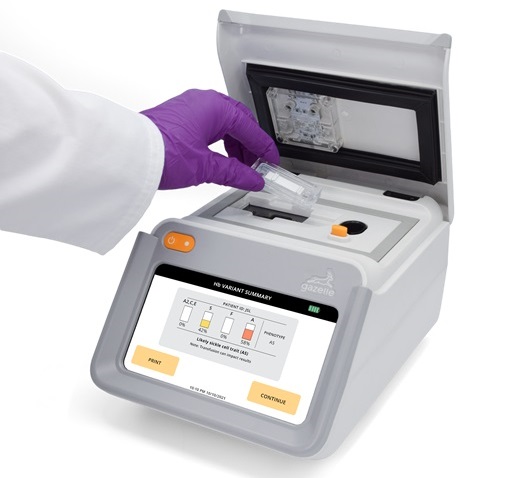
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channel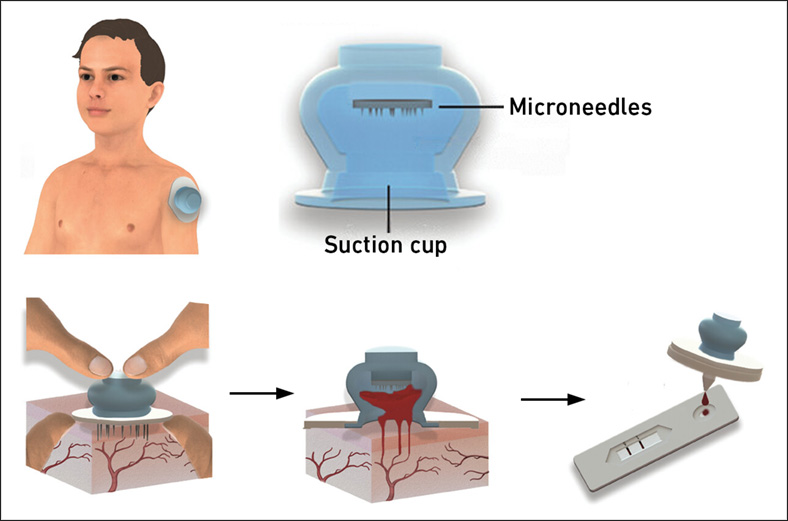
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more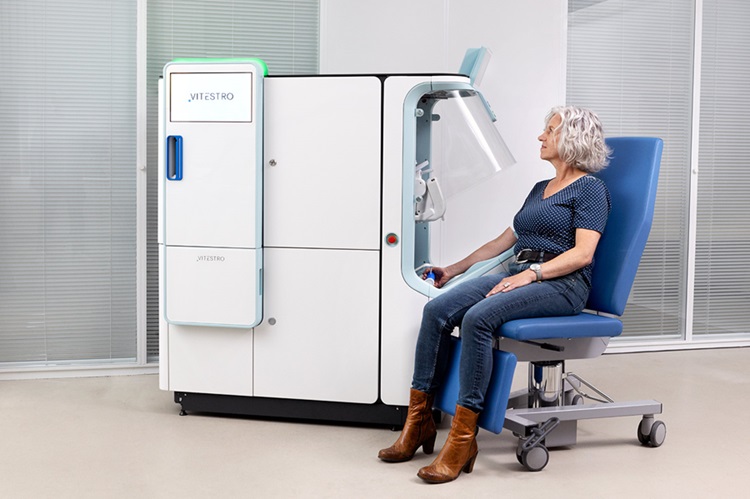
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read more.jpg)
Use of DICOM Images for Pathology Diagnostics Marks Significant Step towards Standardization
Digital pathology is rapidly becoming a key aspect of modern healthcare, transforming the practice of pathology as laboratories worldwide adopt this advanced technology. Digital pathology systems allow... Read more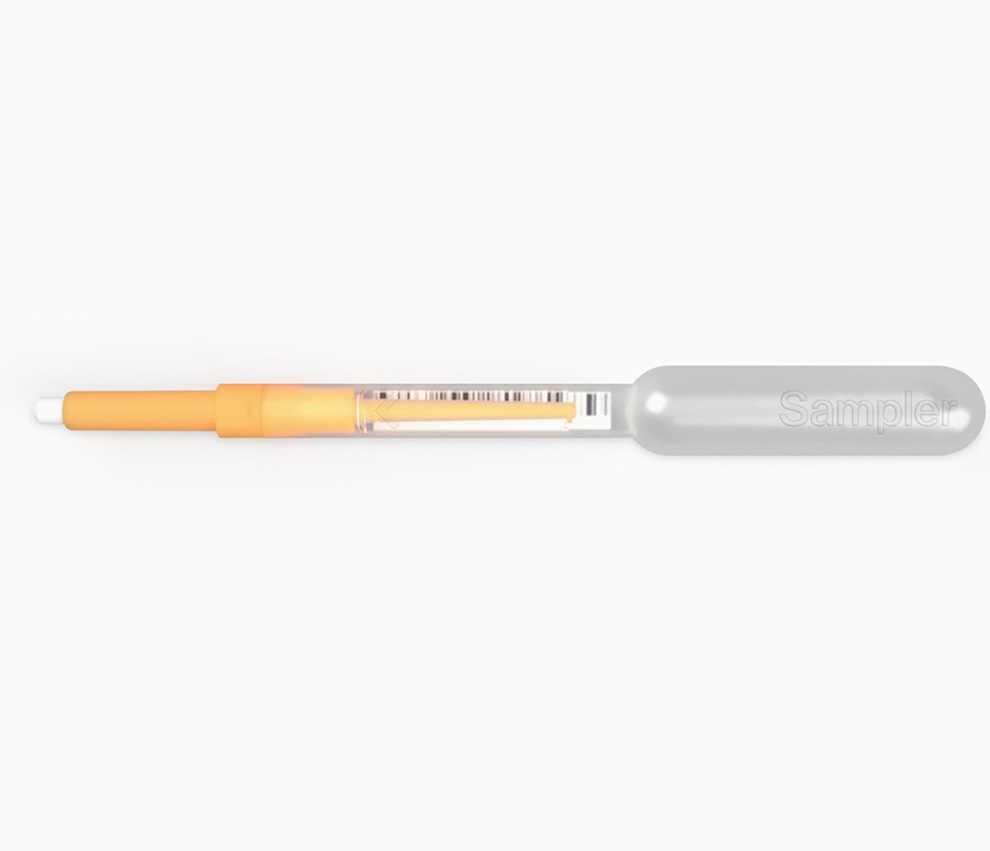
First of Its Kind Universal Tool to Revolutionize Sample Collection for Diagnostic Tests
The COVID pandemic has dramatically reshaped the perception of diagnostics. Post the pandemic, a groundbreaking device that combines sample collection and processing into a single, easy-to-use disposable... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more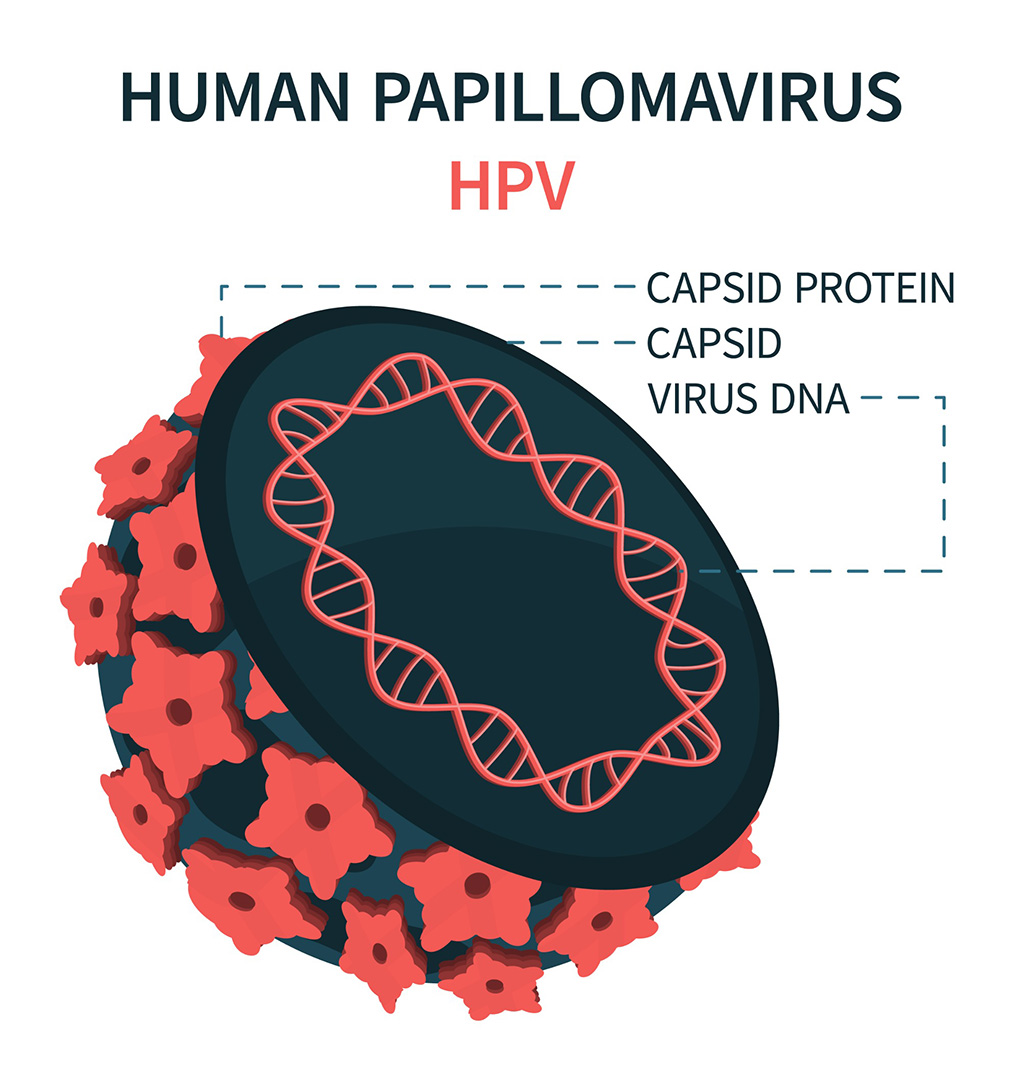
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more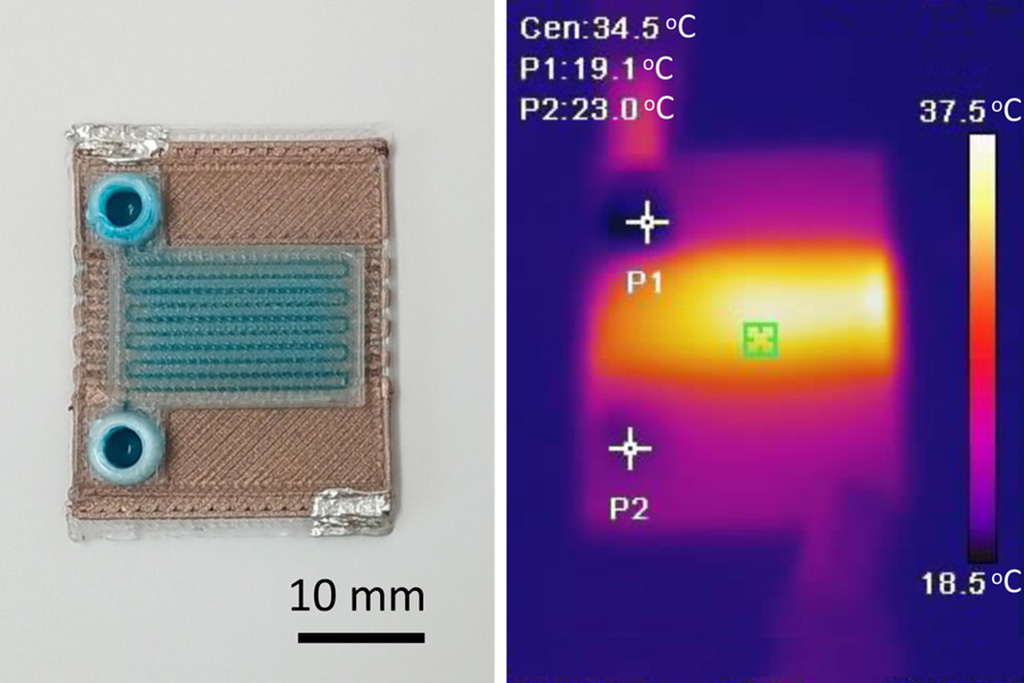
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel_1.jpg)
Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
Thermo Fisher Scientific (Waltham, MA USA) has entered into a strategic distribution agreement with Bio-Techne Corporation (Minneapolis, MN, USA), resulting in a significant collaboration between two industry... Read more
ECCMID Congress Name Changes to ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland) has evolved remarkably. The society is now stronger and broader than ever before... Read more