Molecular Diagnostics
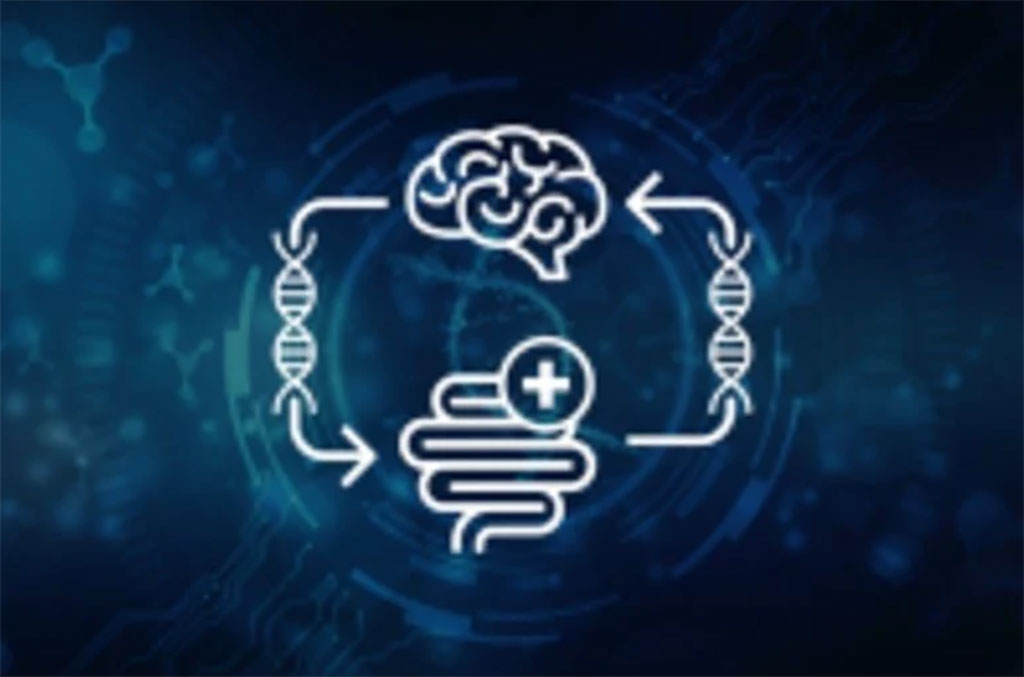
Genetic Link Found Between Gut Disorders and Alzheimer's Disease
Alzheimer’s disease (AD) is the most prevalent form of dementia, characterized by neurodegeneration and a progressive decline in cognitive ability. The disorder ranks as a subject of increasing global public health importance with consequences for wide-ranging social and economic adverse impacts on sufferers, their families, and the society at large. More...02 Aug 2022


BD, CerTest Biotec Announce Commercial Launch of Monkeypox Test
Becton, Dickinson and Company (BD, Franklin Lakes, NJ, USA) and CerTest Biotec (Zaragoza, Spain) have announced their newly developed molecular polymerase chain reaction (PCR) test for the monkeypox virus is now commercially available outside of the US for use in research applications by laboratories. More...31 Jul 2022
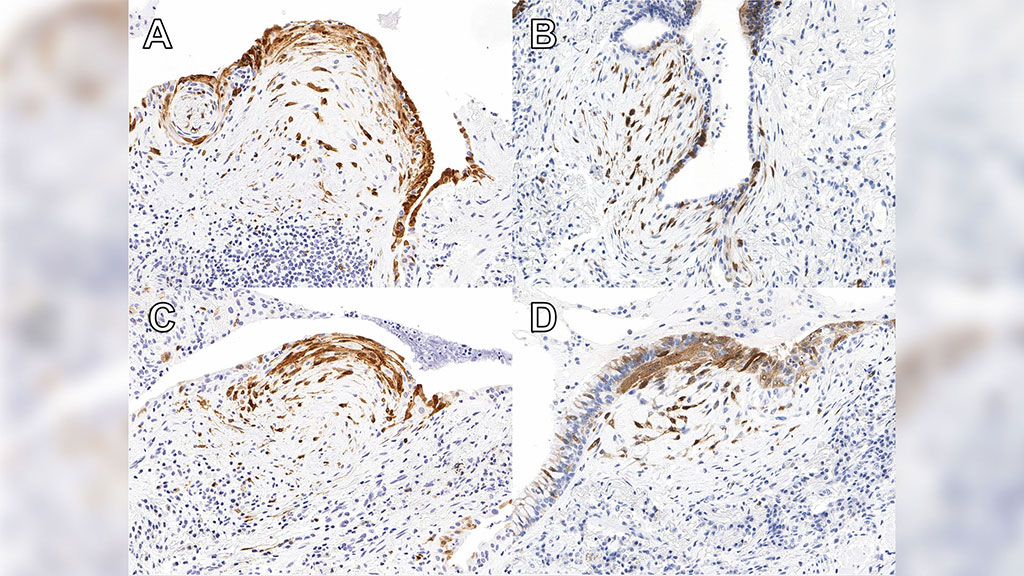
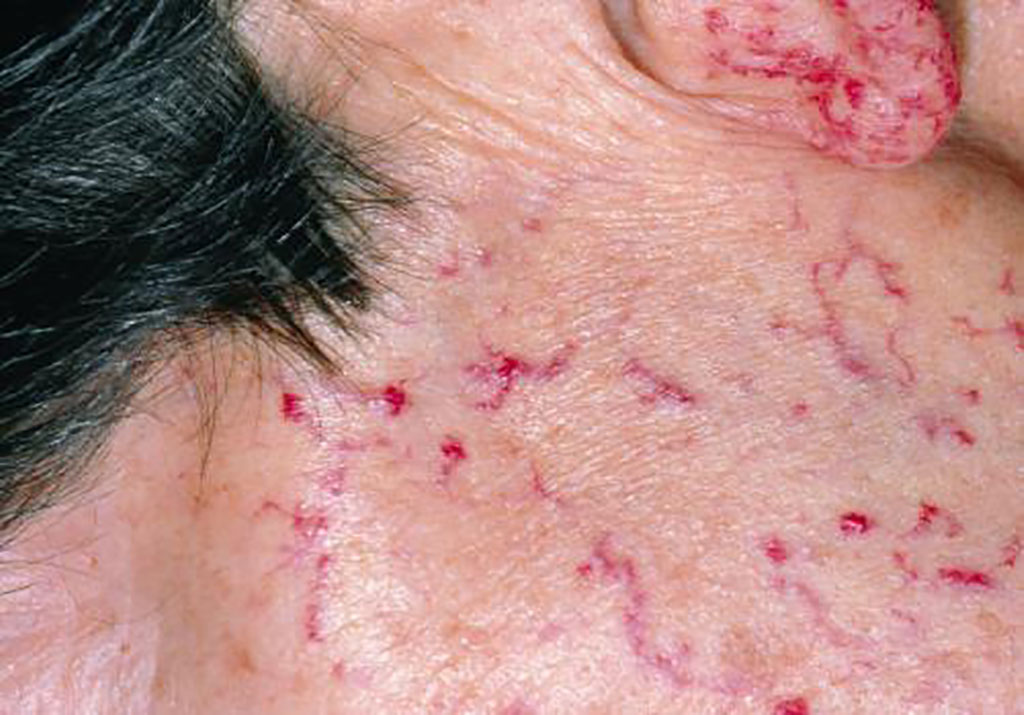
Whole Genome Sequences Discriminate Hereditary Hemorrhagic Telangiectasia Phenotypes
The abnormal vascular structures of hereditary hemorrhagic telangiectasia (HHT) often cause severe anemia due to recurrent hemorrhage, but HHT causal genes do not predict the severity of hematological complications. More...26 Jul 2022
In Other News
Blood Test Detects Multiple Signs of Brain Cancer
Diseases Diagnosed by Isolating Biomarkers in Tears
Blood Test Could Detect Alzheimer’s Up to 17 Years in Advance
First-of-Its-Kind Blood Test Could Transform Cancer Treatment
Novel Assay Quickly and Reliably Detects Prostate Cancer Directly in Blood Samples
DNA Testing Assessed in Childhood Sickle-Cell Anemia Diagnosis
A Non-invasive Skin Test Accurately Diagnoses Covid-19
New Generation Graphene-Based PCT/CRP IVD Test to Give Results Within Minutes
DNA Copy Number Alterations Impact Melanoma Metastasis
Novel Tweak Increases Ability of Liquid Biopsy to Detect Circulating Cancer Cells
New Blood Test Can Identify and Confirm Neurodegenerative Disease
Researchers Create Highly Accurate Non-Invasive Test for Major Liver Diseases
Monkeypox Virus Can Be Detected in Saliva, Semen and Other Clinical Samples
Novel, Urine-Based, Molecular Test Assesses Risk of Aggressive Prostate Cancer
Simple Blood Test May Help Improve Accuracy of Lung Cancer Screenings
Direct-From-Blood Molecular Diagnostic Test Enables Early Detection of Lyme Disease
X chromosome Genes Linked to Male Infertility
New Molecular Assay Tests for Five Respiratory Viruses in Less than Three Hours
Circulating Phosphorylated Tau Proteins Linked to Postoperative Delirium
Novel Liquid Biopsy Platform Could Diagnose Disease State from Any Body Fluid
First of Its Kind Test Detects Gastric Cancer Before Symptoms Appear
Bio-Rad Launches Synthetic Negative and HAI Negative Run Controls
Simple Blood Test Detects Early Liver Cancer in At-Risk Patients
Genetic Testing channel of LabMedica brings the latest in molecular genetics, cytogenetics, and epigenetics, and methods from PCR to FISH, and more.










