Liquid Biopsy Predicts Success of ICI Treatment in Lung Cancer Patients
Posted on 02 Jun 2022
Compared to an invasive tumor biopsy procedure, an extracellular vesicle-based liquid biopsy method was shown to better predict treatment response and survival in patients with non-small cell lung cancer who were undergoing treatment with immune-checkpoint inhibitors (ICIs).
Among the currently approved ICIs are those that target the PD-L1 molecule. PD-L1 binds to PD-1 on the surface of an immune cell, which inhibits immune cell activity. Thus, PD-L1 functions as a key regulator of T-cell activity. It appears that cancer-mediated upregulation of PD-L1 may inhibit T-cells that might otherwise attack tumor cells. Antibodies that bind to either PD-1 or PD-L1 and block their interaction may enable T-cells to attack the tumor.
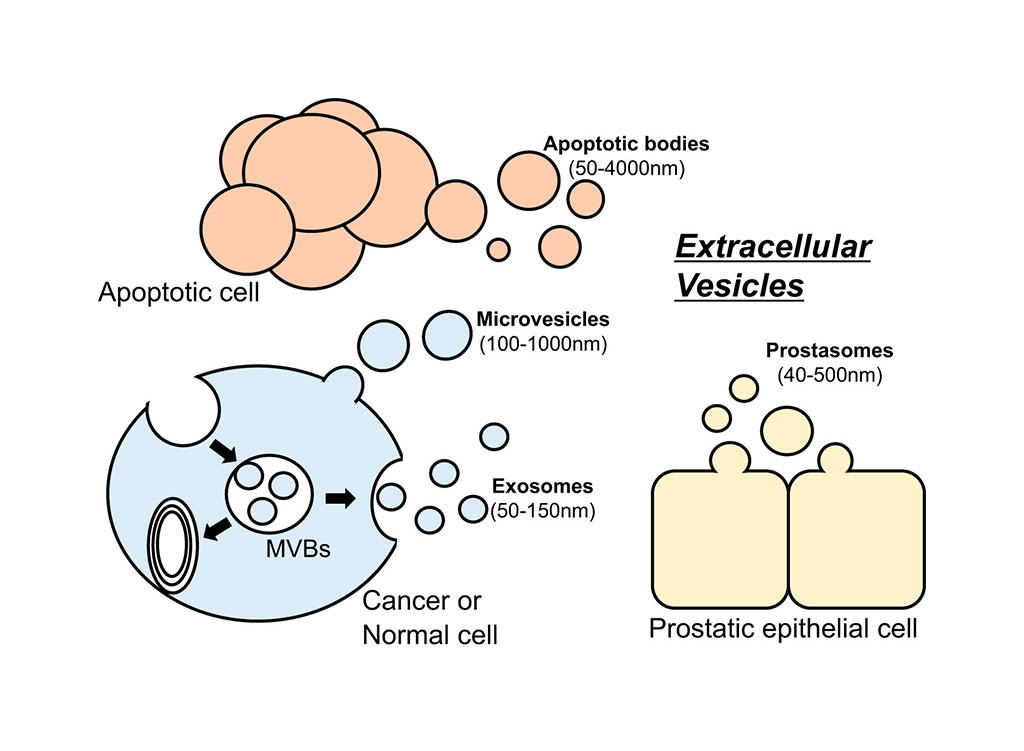
Liquid biopsy is a method for the non-invasive and repeatable analysis of biological material in body fluids and is a promising tool for cancer biomarkers discovery. In particular, there is growing evidence that extracellular vesicles (EVs) play an important role in tumor progression and in tumor-immune interactions.
Extracellular vesicles (EVs) are 40 to 200 micron cell-derived vesicles which play a critical role in cell-to-cell communication, and disease progression. These vesicles, which are present in all biological fluids, contain a wide variety of molecular species such as RNA, DNA, proteins, and lipids from their origin cells, offering a good source of biomarkers. The clinical relevance of EVs has remained largely undetermined, partially owing to challenges in EV analysis. Despite their huge clinical potential, the wide variety of methods for separating EVs from biofluids, which provide material of highly variable purity, and the lack of knowledge regarding methodological reproducibility have impeded the entry of EVs into the clinical arena.
In this regard, investigators at the The Mount Sinai Hospital / Mount Sinai School of Medicine (New York, NY, USA) evaluated whether extracellular vesicle PD-L1 expression could be used as a biomarker for prediction of treatment response and survival in patients with non-small cell lung cancer (NSCLC) undergoing treatment with ICIs.
For this study, the investigators obtained blood samples from two cohorts of 33 and 24 patients with NSCLC receiving ICIs before and at the ninth week of treatment. In addition, a group of 15 patients receiving chemotherapy served as controls. EVs were isolated from the blood samples, and the protein expression of PD-L1 was measured in each group at both time points. Imaging scans of the patients’ tumors before and during treatment were evaluated with an innovative radiomics technology to create a model for prediction of immunotherapy response.
Radiomics is a method that extracts a large number of features from medical images using data-characterization algorithms. These features, termed radiomic features, have the potential to uncover patterns and characteristics present in a tumors that fail to be appreciated by the naked eye and which may be useful for predicting prognosis and therapeutic response for various cancer types.
Results obtained during the current study revealed a decrease in EVs PD-L1 in ICI responders in comparison to non-responders that was an independent biomarker for shorter progression-free survival and overall survival. In contrast, tissue PD-L1 expression, the most commonly used biomarker, was not predictive for either durable response or survival.
“These results will have an impact in the search for biomarkers to predict for immunotherapy outcome in patients with lung cancer as no truly reliable biomarkers have been found yet,” said senior author Dr. Christian Rolfo, professor of medicine at The Mount Sinai School of Medicine and president of the International Society of Liquid Biopsy. “If validated in larger prospective cohorts of patients, as we are working on now, this protein could complement or substitute for the tissue PD-L1 as the standard of care in these and other types of tumor patients receiving immunotherapy, especially because it is minimally invasive and can be repetitive during treatment, being able to detect changes in the tumor during the treatment in real time.”
The study was published in June 2, 2022, online edition of the Journal of Experimental & Clinical Cancer Research.
Related Links:
The Mount Sinai Hospital / Mount Sinai School of Medicine