Detection of ctDNA Can Guide Post-Surgical Treatment of Colon Cancer Patients
Posted on 09 Jun 2022
Circulating tumor DNA (ctDNA) has been shown to be a biomarker that can predict whether, following surgical removal of the tumor, a stage II colon cancer patient can safely forgo chemotherapy without risk of recurrence of the disease
Stage II colon cancer is defined as a cancer that has grown through the wall of the colon, but does not extend to the lymph nodes or other organs. While most patients with stage II colon cancer are cured after surgery to remove the cancer from the bowel, the cancer will recur in around 20% of patients. Currently, [at least in Australia] chemotherapy, with its frequent unpleasant side-effects, is offered to all stage II colon cancer patients despite a majority not actually needing it.
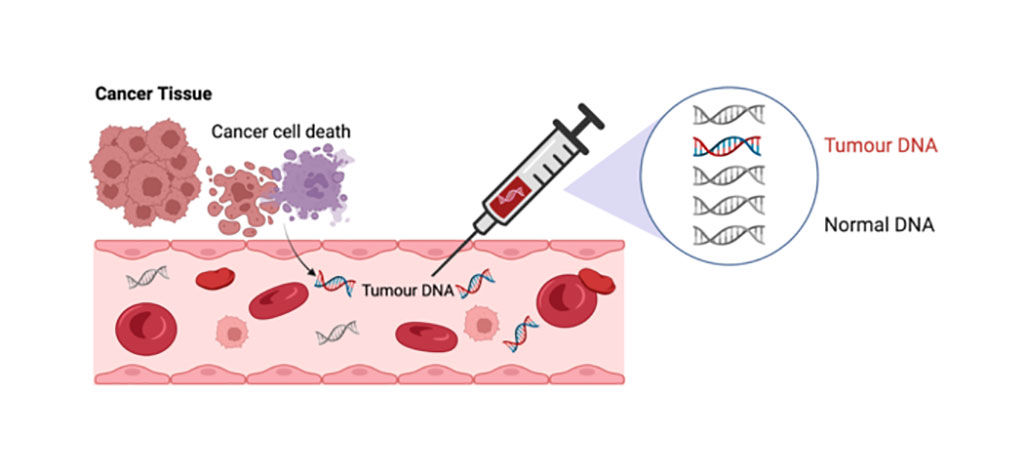
Previous studies have demonstrated that the presence of ctDNA after surgery to remove a tumor from the colon predicted very poor recurrence-free survival, whereas its absence predicted a low risk of recurrence. Based on these findings, investigators at the Walter and Eliza Hall Institute (Melbourne, Australia) and Johns Hopkins University (Baltimore, MD, USA) conducted a trial to assess whether a ctDNA-guided approach could reduce the use of chemotherapy without compromising recurrence risk.
In this study, 455 stage II colon cancer patients were randomly assigned to ctDNA-guided management (302 patients) or to standard management (153 patients). For ctDNA-guided management, a ctDNA-positive result at four or seven weeks after surgery prompted oxaliplatin-based or fluoropyrimidine chemotherapy. Patients who were ctDNA-negative were not treated.
Results revealed that 37 months after surgery a lower percentage of patients in the ctDNA-guided group than in the standard-management group received chemotherapy (15% vs. 28%). In the evaluation of two-year recurrence-free survival, ctDNA-guided management was as effective as standard management (93.5% and 92.4%, respectively; absolute difference, 1.1%). Three-year recurrence-free survival was 86.4% among ctDNA-positive patients who received adjuvant chemotherapy and 92.5% among ctDNA-negative patients who did not.
First author, Dr. Jeanne Tie, associate professor of medical oncology at the Walter and Eliza Hall Institute, said, “We found that when a patient’s blood test does not reveal ctDNA after colon surgery, the likelihood of micrometastases is very low and chemotherapy can be avoided as there are no tumor fragments left to kill. Our trial has conclusively shown how the ctDNA blood test can be used to direct post-surgical therapy in stage II colon cancer and substantially reduce the number of patients treated with chemotherapy, without impacting the risk of cancer relapse. While chemotherapy can be essential and lifesaving, many patients are receiving the treatment and its associated toxicities without any benefit.”
The ctDNA study was published in the June 4, 2022, online edition of The New England Journal of Medicine.
Related Links:
Walter and Eliza Hall Institute
Johns Hopkins University