Fujirebio and Agappe Collaborate on CLIA-Based Immunoassay
|
By LabMedica International staff writers Posted on 22 Jan 2024 |

Fujirebio Holdings (Tokyo, Japan) and Agappe Diagnostics (Kochi, India) have entered into a Contract Development and Manufacturing Organization (CDMO) partnership for manufacturing cartridge-based CLIA system reagents for the Mispa i60 and Mispa i121 immunology analyzers. These analyzers and reagents will be sold under Agappe’s brand, making it the first Indian local company with a complete chemiluminescence solution made from reagents produced domestically.
The companies have outlined plans to launch these products in June 2024. Significant milestones have already been achieved, including the finalization of a supply and license agreement in March 2023. Agappe is currently in the advanced stages of technical transfer from Fujirebio, which will enable the Indian company to manufacture chemiluminescence reagents using Fujirebio’s technology and materials. The collaboration is set to make a substantial impact, with phased launches planned for a complete range of over 30 parameters, spanning various medical segments such as oncology, thyroid, cardiac, fertility, and infectious diseases. Additionally, Fujirebio’s groundbreaking biomarkers for Alzheimer’s and other neurodegenerative diseases are also included in this project.
Under this agreement, Fujirebio will be responsible for supplying the Mispa i60 and i121 analyzers and the raw materials for the reagents. Agappe, on the other hand, will focus on developing and manufacturing reagents specifically tailored for these instruments, ensuring compliance with all regulatory requirements. This partnership is based on a shared vision of leveraging each company’s unique strengths to not only foster growth in India's immunoassay market but also to significantly contribute to the advancement and enhancement of immunoassay solutions within the Indian healthcare sector.
“This partnership with Agappe, the renowned market leader with years of success in clinical chemistry, hematology, immunochemistry, and point of care in India, will accelerate our CDMO strategy in one of the most important and fastest growing countries in the world,” said Goki Ishikawa, President and CEO, Fujirebio Holdings, Inc. “We share a vision that, combining mutually complementary strengths of both companies, Agappe together with Fujirebio can deliver high-quality chemiluminescence solutions with cost competitiveness and contribute to the healthcare advancement in India.”
“Leveraging the combined strengths of Agappe and Fujirebio, we are committed to introducing high quality, affordable, and unique chemiluminescence solutions for the country,” added Thomas John, Managing Director, Agappe Diagnostics, Ltd. “This partnership symbolizes our dedication to innovation and our pledge to make advanced diagnostic products more accessible to every Indian. We are poised to set new benchmarks in the industry, emphasizing our joint endeavor to enhance healthcare standards across our nation."
Related Links:
Fujirebio Holdings
Agappe Diagnostics
Latest Industry News
- WHX Labs in Dubai spotlights leadership skills shaping next-generation laboratories
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- WHX Labs Dubai to Gather Global Experts in Antimicrobial Resistance at Inaugural AMR Leaders’ Summit
- BD and Penn Institute Collaborate to Advance Immunotherapy through Flow Cytometry
- Abbott Acquires Cancer-Screening Company Exact Sciences
- Roche and Freenome Collaborate to Develop Cancer Screening Tests
- Co-Diagnostics Forms New Business Unit to Develop AI-Powered Diagnostics
- Qiagen Acquires Single-Cell Omics Firm Parse Biosciences
- Puritan Medical Products Showcasing Innovation at AMP2025 in Boston
- Advanced Instruments Merged Under Nova Biomedical Name
- Bio-Rad and Biodesix Partner to Develop Droplet Digital PCR High Complexity Assays
- Hologic to be Acquired by Blackstone and TPG
- Bio-Techne and Oxford Nanopore to Accelerate Development of Genetics Portfolio
- Terumo BCT and Hemex Health Collaborate to Improve Access to Testing for Hemoglobin Disorders
Channels
Clinical Chemistry
view channel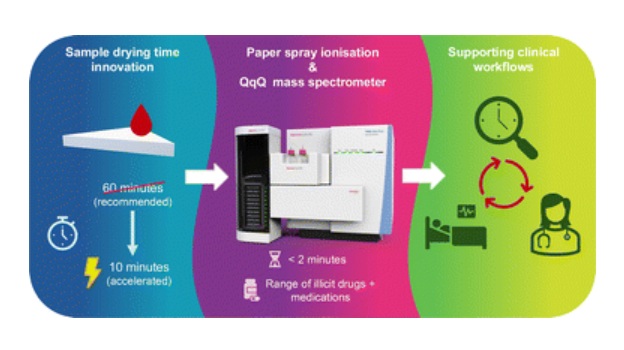
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read more
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read moreMolecular Diagnostics
view channel
Genetic Test Could Improve Early Detection of Prostate Cancer
Prostate cancer is the second-leading cause of cancer deaths among men in the United States and remains a major health burden. Current screening with prostate-specific antigen (PSA) blood tests can sometimes... Read more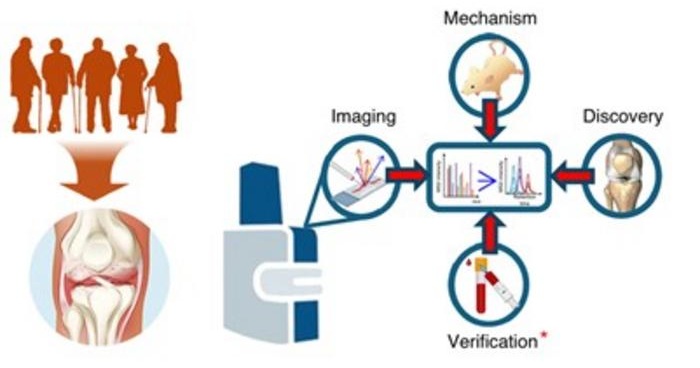
Bone Molecular Maps to Transform Early Osteoarthritis Detection
Osteoarthritis affects more than 500 million people worldwide and is a major cause of pain, disability, and reduced quality of life. By the time it is diagnosed through symptoms and visible cartilage loss,... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more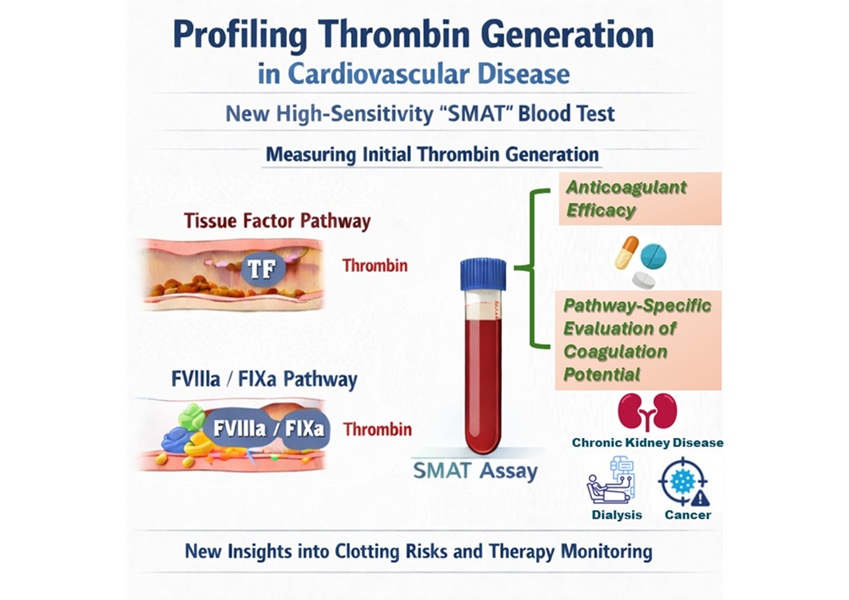
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel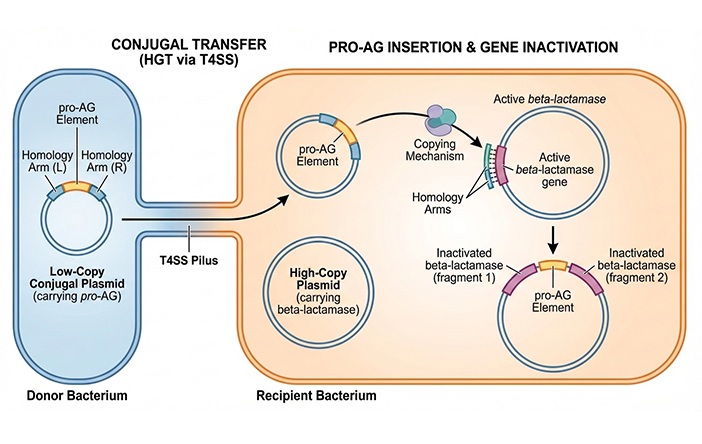
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
Malaria remains one of the world’s deadliest infectious diseases, killing hundreds of thousands each year, mostly in under-resourced regions where laboratory infrastructure is limited. Diagnosis still... Read more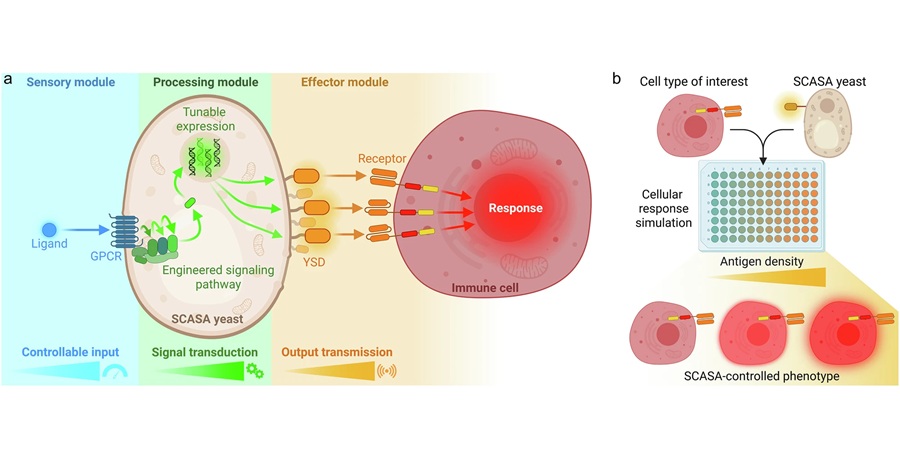
Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Developing new cancer immunotherapies is a slow, costly, and high-risk process, particularly for CAR T cell treatments that must precisely recognize cancer-specific antigens. Small differences in tumor... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more