Menstrual Blood Testing Technology Eliminates Need for Finger Prick or Lab Blood Draw
|
By LabMedica International staff writers Posted on 11 Jan 2024 |
Traditional methods of blood testing require invasive procedures administered by medical professionals, and can be time-consuming as well as expensive. Not everyone has the time, access, and financial means to get laboratory results for blood work, however, billions of women globally have their period every single month. Until now, menstrual samples had never previously been explored as a diagnostic source for health information. Now, for the first time, a safe and simple technology allows menstrual blood samples to be used as an alternative to traditionally collected venous blood draws. This breakthrough provides an opportunity for testing important biomarkers for millions of menstruating women across the world.
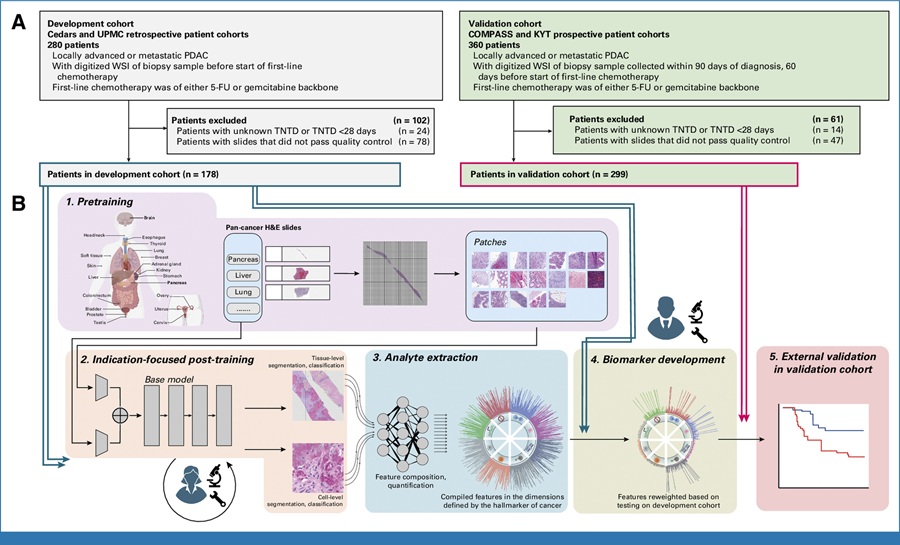
Qvin (Menlo Park, CA, USA) has proved the clinical relevancy of menstrual blood for several important biomarkers. The company’s Q-Pad technology uses menstrual blood to gain health insights in a non-invasive, convenient manner and can be used for testing anywhere in the world. The Q-Pad was initially created to identify biomarkers for HPV and has expanded and identified additional biomarkers to test for, including pre/diabetes, anemia, fertility, perimenopause, endometriosis, and thyroid health. Each Q-Pad includes a removable strip; once the Q-Pad has sufficiently collected a menstrual sample, the removed collection strip is sent to a CLIA-Certified (Clinical Laboratory Improvement Amendments) laboratory for clinical testing. Users receive their results via the free and convenient Qvin app.
Q-Pad allows specimens to be submitted directly to the lab and receive reports on key health conditions that often go undiagnosed or misdiagnosed including pre/diabetes, anemia, fertility, perimenopause, endometriosis, and thyroid health. For instance, women seeking to understand their fertility status can soon monitor various reproductive hormones via menstrual blood using the Q-Pad. Qvin also offers the A1c Q-Pad Test Kit which measures the average blood sugar over a three-month period by testing the A1c biomarker for people with diabetes. Qvin, in collaboration with researchers at academic institutions, has published peer-reviewed research validating other biomarkers that can also be monitored. Both the Q-Pad and A1c Q-Pad Test Kit have received US FDA clearance.
"With the first ever FDA-cleared menstrual blood health test, Qvin is paving the way to important new opportunities for women's health and this is just the beginning," said Dr. Sara Naseri, Qvin Co-founder. "We are simplifying routine testing, and freeing up resources that can be used on providing care and ultimately our goal is to make health care much more accessible."
Latest Clinical Chem. News
- AI Sensor Detects Neurological Disorders Using Single Saliva Drop
- New Blood Test Index Offers Earlier Detection of Liver Scarring
- Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
- Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
- Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
- Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
- New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
- Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
- Study Compares Analytical Performance of Quantitative Hepatitis B Surface Antigen Assays
- Blood Test Could Predict and Identify Early Relapses in Myeloma Patients
- Compact Raman Imaging System Detects Subtle Tumor Signals
- Noninvasive Blood-Glucose Monitoring to Replace Finger Pricks for Diabetics
- POC Breath Diagnostic System to Detect Pneumonia-Causing Pathogens
- Online Tool Detects Drug Exposure Directly from Patient Samples
- Chemical Imaging Probe Could Track and Treat Prostate Cancer
- Mismatch Between Two Common Kidney Function Tests Indicates Serious Health Problems
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read more
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Agilent Technologies Acquires Pathology Diagnostics Company Biocare Medical
Agilent Technologies (Santa Clara, CA, USA) has entered into a definitive agreement to acquire Biocare Medical (Pacheco, CA, USA), expanding its pathology portfolio through the addition of highly complementary... Read more
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more