Rapid Lateral Flow Assays Detect COVID-19 Variants and Differentiate COVID-19 from Other Respiratory Viral Diseases
|
By LabMedica International staff writers Posted on 02 Mar 2021 |
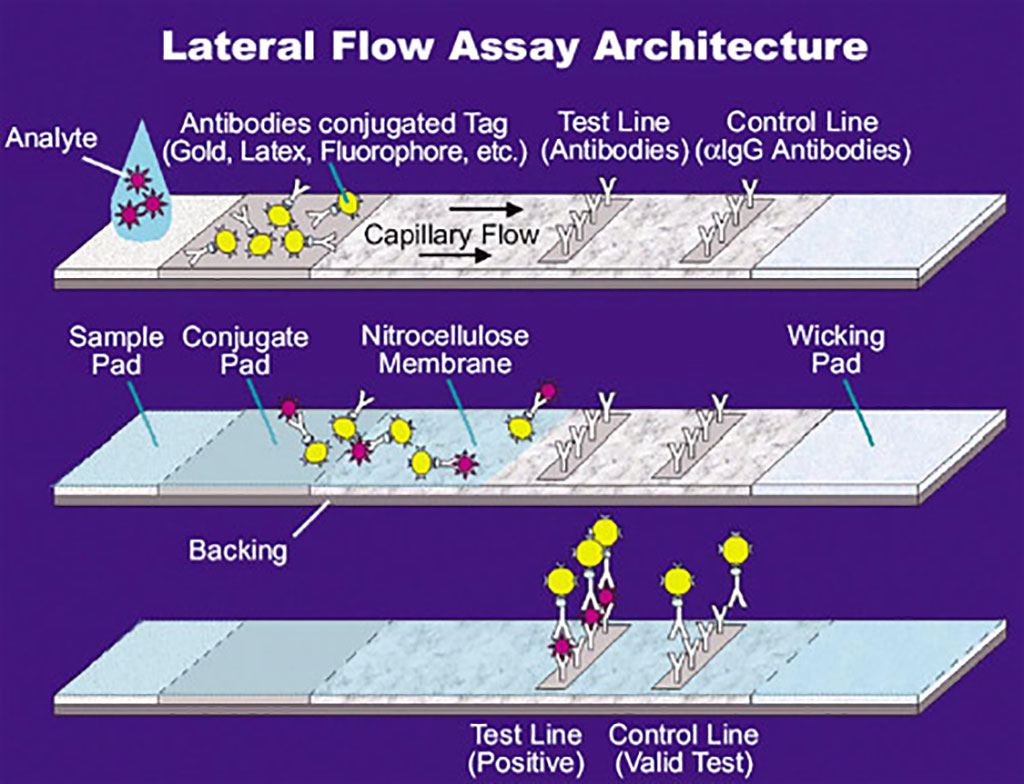
Image: Illustration of a lateral flow assay (LFA) (Photo courtesy of U.S. National Aeronautics and Space Administration via Wikimedia Commons)
A recent publication reported the development of two rapid diagnostic tests - one that detects COVID-19 variants and one that differentiates COVID-19 from other respiratory viral diseases.
Investigators at the University of Minnesota Medical School (Minneapolis/St.Paul, USA) used the CRISPR/Cas9 gene editing tool to develop two rapid lateral flow diagnostic tests. CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. Since 2013, the CRISPR/Cas9 system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs (sgRNAs) into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system from Streptococcus pyogenes is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides that shepherd the Cas9 protein to the target gene on a DNA strand.
The investigators integrated commercially available reagents into a CRISPR/Cas9-based lateral flow assay (LFA) that could detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequences with single-base specificity. This approach required minimal equipment and represented a simplified platform for field-based deployment. They also developed a rapid, multiplex fluorescence CRISPR/Cas9 nuclease cleavage assay capable of detecting and differentiating SARS-CoV-2, influenza A and B, and respiratory syncytial virus (RSV) in a single reaction.
The LFA test strips employed bound fluorescein isothiocyanate (FITC)/6-Carboxyfluorescein (FAM) and biotin to generate a positive result. Therefore, the investigators used a FITC/FAM-labeled PCR primer and a nuclease inactive (“dead”) biotinylated Cas9 and a single sgRNA specific for the ORF8a gene of SARS-Co-V-2 to label amplicons for detection by LFA. This approach was capable of single-nucleotide resolution and avoided false positives from primer dimer or non-specific amplification artifacts that could occur with the use of tandem FITC- and biotin-labeled primers for LFA.
"The approval of the SARS-CoV-2 vaccine is highly promising, but the time between first doses and population immunity may be months," said first author Dr. Mark J. Osborn, assistant professor of pediatrics at the University of Minnesota Medical School. "This testing platform can help bridge the gap between immunization and immunity."
The rapid LFA tests were described in the February 12, 2021, online edition of the journal Bioengineering.
Related Links:
University of Minnesota Medical School
Investigators at the University of Minnesota Medical School (Minneapolis/St.Paul, USA) used the CRISPR/Cas9 gene editing tool to develop two rapid lateral flow diagnostic tests. CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. Since 2013, the CRISPR/Cas9 system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs (sgRNAs) into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system from Streptococcus pyogenes is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides that shepherd the Cas9 protein to the target gene on a DNA strand.
The investigators integrated commercially available reagents into a CRISPR/Cas9-based lateral flow assay (LFA) that could detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequences with single-base specificity. This approach required minimal equipment and represented a simplified platform for field-based deployment. They also developed a rapid, multiplex fluorescence CRISPR/Cas9 nuclease cleavage assay capable of detecting and differentiating SARS-CoV-2, influenza A and B, and respiratory syncytial virus (RSV) in a single reaction.
The LFA test strips employed bound fluorescein isothiocyanate (FITC)/6-Carboxyfluorescein (FAM) and biotin to generate a positive result. Therefore, the investigators used a FITC/FAM-labeled PCR primer and a nuclease inactive (“dead”) biotinylated Cas9 and a single sgRNA specific for the ORF8a gene of SARS-Co-V-2 to label amplicons for detection by LFA. This approach was capable of single-nucleotide resolution and avoided false positives from primer dimer or non-specific amplification artifacts that could occur with the use of tandem FITC- and biotin-labeled primers for LFA.
"The approval of the SARS-CoV-2 vaccine is highly promising, but the time between first doses and population immunity may be months," said first author Dr. Mark J. Osborn, assistant professor of pediatrics at the University of Minnesota Medical School. "This testing platform can help bridge the gap between immunization and immunity."
The rapid LFA tests were described in the February 12, 2021, online edition of the journal Bioengineering.
Related Links:
University of Minnesota Medical School
Latest Molecular Diagnostics News
- World’s First Portable POC Test Simultaneously Detects Four Common STIs in One Hour
- Simple One-Hour Saliva Test Detects Common Cancers
- Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
- Blood Test Could Spot Common Post-Surgery Condition Early
- New Blood Test Can Help Predict Testicular Cancer Recurrence
- New Test Detects Alzheimer’s by Analyzing Altered Protein Shapes in Blood
- New Diagnostic Markers for Multiple Sclerosis Discovered in Cerebrospinal Fluid
- Cell-Free DNA Predicts Bloodstream Infections in Children with Leukemia
- Study Uses Blood Samples to Identify Diseases Years Before They Start
- MicroRNA-Based Method Predicts CKD and Cardiovascular Risk
- Swab Test Helps Transplant Patients Receive Right Anti-Rejection Medication Dose
- Blood Test Predicts Which Bladder Cancer Patients May Safely Skip Surgery
- Ultra-Sensitive DNA Test Identifies Relapse Risk in Aggressive Leukemia
- Blood Test Could Help Detect Gallbladder Cancer Earlier
- New Blood Test Score Detects Hidden Alcohol-Related Liver Disease
- New Blood Test Predicts Who Will Most Likely Live Longer
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read more
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more