Liquid Biopsy Lung Cancer Screening Method Developed
|
By LabMedica International staff writers Posted on 09 Apr 2020 |
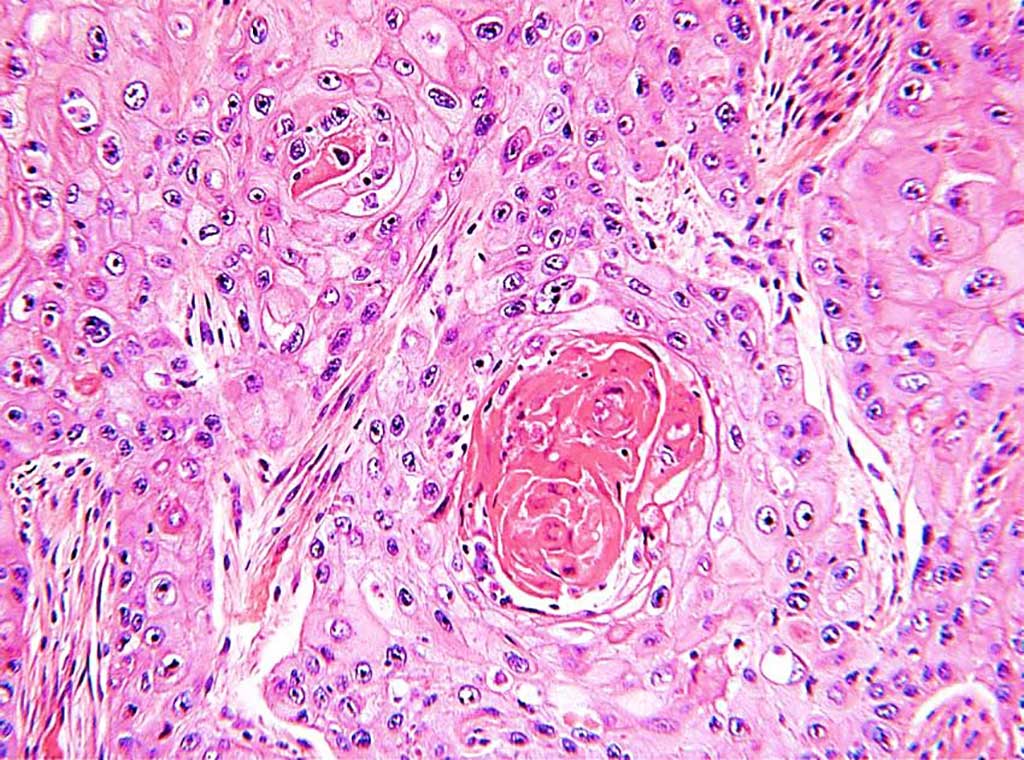
Image: Histological sections of a moderately well differentiated squamous cell carcinoma of the lung showing infiltrating sheets and tongues of malignant squamous cells with whorls of keratin (Photo courtesy of Department of Health Western Australia).
Genomic blood tests for cancer screening and early detection have become the focus of attention in the molecular diagnostic space, though most activity so far has been either toward pan-cancer screening tools, or in a few other specific tumor types like colorectal cancer, where tests hope to vie against colonoscopy and existing stool-based methods.
Radiologic screening of high-risk adults reduces lung-cancer-related mortality; however, a small minority of eligible individuals undergo such screening in the USA. The availability of blood-based tests could increase screening uptake. A novel circulating tumor DNA (ctDNA) detection assay has been developed that could help physicians screen individuals at risk for lung cancer.
A large team of scientists at Stanford University (Stanford, CA, USA) and their colleagues have described a method, called Lung-CLiP (lung cancer likelihood in plasma), involves targeted sequencing of cell-free DNA (cfDNA) from plasma and matched white blood cell DNA to assess copy number and single nucleotide variants, coupled with a machine learning model that estimates the probability that a cfDNA mutation is tumor-derived. The estimate is based on biological and technical features specific to each variant, such as background frequency, cfDNA fragment size, the gene affected, and the likelihood of clonal hematopoiesis.
The team first trained and optimized Lung-CLiP in an initial sample of 104 patients with early stage non-small cell lung cancer and 56 matched controls. When they then applied it to an independent set of validation samples (46 cases and 48 risk-matched controls), the test was able to discriminate early-stage lung cancer patients with sensitivity and specificity levels that the authors believe suggest a significant benefit to the clinic: depending on where they set their specificity threshold, the method could achieve 63% sensitivity for stage I tumors and up to 75% sensitivity in detecting patients with stage III disease. Setting their cutoff at 98% specificity, the investigators found that Lung-CLiP detected 41% of patients with stage I disease, 54% of patients with stage II disease and 67% of those with stage III disease.
Ash Alizadeh, MD, PhD, an associate professor of Oncology and a senior author of the study, said, “Lung-CLiP could help increase the rate of early detection. This would be analogous to how stool-based testing proposes to improve screening for colorectal cancers, especially in populations where adoption of colonoscopy is lower than currently recommended.” The study was published on March 25, 2020 in the journal Nature.
Related Links:
Stanford University
Radiologic screening of high-risk adults reduces lung-cancer-related mortality; however, a small minority of eligible individuals undergo such screening in the USA. The availability of blood-based tests could increase screening uptake. A novel circulating tumor DNA (ctDNA) detection assay has been developed that could help physicians screen individuals at risk for lung cancer.
A large team of scientists at Stanford University (Stanford, CA, USA) and their colleagues have described a method, called Lung-CLiP (lung cancer likelihood in plasma), involves targeted sequencing of cell-free DNA (cfDNA) from plasma and matched white blood cell DNA to assess copy number and single nucleotide variants, coupled with a machine learning model that estimates the probability that a cfDNA mutation is tumor-derived. The estimate is based on biological and technical features specific to each variant, such as background frequency, cfDNA fragment size, the gene affected, and the likelihood of clonal hematopoiesis.
The team first trained and optimized Lung-CLiP in an initial sample of 104 patients with early stage non-small cell lung cancer and 56 matched controls. When they then applied it to an independent set of validation samples (46 cases and 48 risk-matched controls), the test was able to discriminate early-stage lung cancer patients with sensitivity and specificity levels that the authors believe suggest a significant benefit to the clinic: depending on where they set their specificity threshold, the method could achieve 63% sensitivity for stage I tumors and up to 75% sensitivity in detecting patients with stage III disease. Setting their cutoff at 98% specificity, the investigators found that Lung-CLiP detected 41% of patients with stage I disease, 54% of patients with stage II disease and 67% of those with stage III disease.
Ash Alizadeh, MD, PhD, an associate professor of Oncology and a senior author of the study, said, “Lung-CLiP could help increase the rate of early detection. This would be analogous to how stool-based testing proposes to improve screening for colorectal cancers, especially in populations where adoption of colonoscopy is lower than currently recommended.” The study was published on March 25, 2020 in the journal Nature.
Related Links:
Stanford University
Latest Pathology News
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
- First-Of-Its-Kind Test Identifies Autism Risk at Birth
- AI Algorithms Improve Genetic Mutation Detection in Cancer Diagnostics
- Skin Biopsy Offers New Diagnostic Method for Neurodegenerative Diseases
- Fast Label-Free Method Identifies Aggressive Cancer Cells
- New X-Ray Method Promises Advances in Histology
- Single-Cell Profiling Technique Could Guide Early Cancer Detection
- Intraoperative Tumor Histology to Improve Cancer Surgeries
- Rapid Stool Test Could Help Pinpoint IBD Diagnosis
Channels
Clinical Chemistry
view channel
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read more
Rapid Blood Testing Method Aids Safer Decision-Making in Drug-Related Emergencies
Acute recreational drug toxicity is a frequent reason for emergency department visits, yet clinicians rarely have access to confirmatory toxicology results in real time. Instead, treatment decisions are... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Rapid Test Promises Faster Answers for Drug-Resistant Infections
Drug-resistant pathogens continue to pose a growing threat in healthcare facilities, where delayed detection can impede outbreak control and increase mortality. Candida auris is notoriously difficult to... Read more
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read morePathology
view channel
Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, in part because of its dense tumor microenvironment that influences how tumors grow and respond to treatment.... Read more
New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
Sputum smear microscopy remains central to tuberculosis treatment monitoring and follow-up, particularly in high‑burden settings where serial testing is routine. Yet consistent, repeatable bacillary assessment... Read more
AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
Stem cell and bone marrow transplants can be lifesaving, but serious complications may arise months after patients leave the hospital. One of the most dangerous is chronic graft-versus-host disease, in... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreIndustry
view channel
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more