Rare Infection in Transplant Recipients Linked to Donors
|
By LabMedica International staff writers Posted on 01 Jun 2017 |
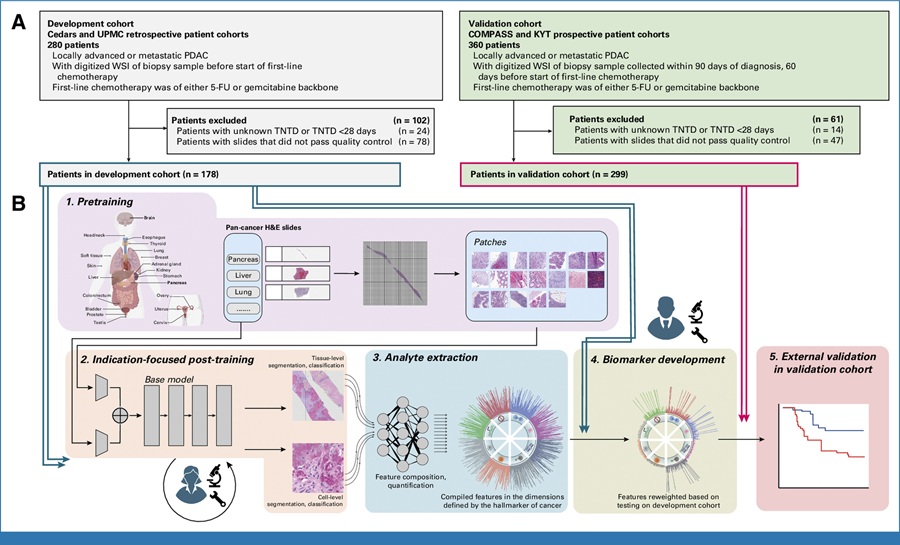
Image: Mycoplasma hominis, the bacteria responsible for a rare infection in transplant recipients (Photo courtesy of the Mayo Clinic).
The way in which heart and lung transplant recipients acquired a specific species of bacteria, Mycoplasma hominis, had been previously undefined, and the bacterium was difficult to test for and detect. Originally, this bacterium was considered to reside exclusively in, and be a potential pathogen of the area of the reproductive and urinary organs.
M. hominis resists most antibiotics, and the three antibiotic treatment recommendations for these infections are neither standard for post-transplant recipient care nor are they standard in therapy regimens for surgical site infections. Heart and lung transplant recipient infection caused by M. hominis may present with, surgical site infection and mediastinitis, which is an inflammation of tissue in mid-chest.
Scientists at the Mayo Clinic investigated lung and heart-lung transplants between 1998 and July 2015 at the Mayo Clinic. Seven previously unreported cases of transplant recipients with M. hominis infection were discovered. In each case, pre-transplant sputum cultures had tested negative for M. hominis. A literature review since 1950 found 15 cases of M. hominis infection in lung, heart or heart-lung transplant recipients. The way the bacteria spread remained uncertain. Given its normal residence in the genitourinary tract, some speculated that infection arose from urinary catheter placement during the transplant surgery.
Bronchoalveolar lavage samples were submitted for detecting organisms commonly found in immunocompromised hosts undergoing clinically indicated bronchoscopy were tested for M. hominis DNA. DNA was extracted on the MagNA Pure LC instrument using the MagNA Pure total nucleic acid isolation kit. Common testing methods have proven insufficient in identifying the bacteria, but the use of polymerase chain reaction offered a more time-sensitive and specific test for the bacteria. With this method, the scientists were able to focus in on a certain portion of DNA and then create multiple copies to amplify the segment. Polymerase chain reaction detection reduces the time to detect M. hominis to a few hours, compared to the two to five days needed for a culture media test.
Mark E. Wylam, MD, the lead investigator said, “These findings could affect how we approach the evaluation of organ donors. If potential transmission of these harmful bacteria can be identified and addressed, the recipient will face a decreased risk of infection and its serious complications. This study shows us that surveillance of both donor and recipient are important in recognizing M. hominis and the infection it can cause.” The study was published in the May 2017 issue of the journal EbioMedicine.
M. hominis resists most antibiotics, and the three antibiotic treatment recommendations for these infections are neither standard for post-transplant recipient care nor are they standard in therapy regimens for surgical site infections. Heart and lung transplant recipient infection caused by M. hominis may present with, surgical site infection and mediastinitis, which is an inflammation of tissue in mid-chest.
Scientists at the Mayo Clinic investigated lung and heart-lung transplants between 1998 and July 2015 at the Mayo Clinic. Seven previously unreported cases of transplant recipients with M. hominis infection were discovered. In each case, pre-transplant sputum cultures had tested negative for M. hominis. A literature review since 1950 found 15 cases of M. hominis infection in lung, heart or heart-lung transplant recipients. The way the bacteria spread remained uncertain. Given its normal residence in the genitourinary tract, some speculated that infection arose from urinary catheter placement during the transplant surgery.
Bronchoalveolar lavage samples were submitted for detecting organisms commonly found in immunocompromised hosts undergoing clinically indicated bronchoscopy were tested for M. hominis DNA. DNA was extracted on the MagNA Pure LC instrument using the MagNA Pure total nucleic acid isolation kit. Common testing methods have proven insufficient in identifying the bacteria, but the use of polymerase chain reaction offered a more time-sensitive and specific test for the bacteria. With this method, the scientists were able to focus in on a certain portion of DNA and then create multiple copies to amplify the segment. Polymerase chain reaction detection reduces the time to detect M. hominis to a few hours, compared to the two to five days needed for a culture media test.
Mark E. Wylam, MD, the lead investigator said, “These findings could affect how we approach the evaluation of organ donors. If potential transmission of these harmful bacteria can be identified and addressed, the recipient will face a decreased risk of infection and its serious complications. This study shows us that surveillance of both donor and recipient are important in recognizing M. hominis and the infection it can cause.” The study was published in the May 2017 issue of the journal EbioMedicine.
Latest Microbiology News
- Study Highlights Accuracy Gaps in Consumer Gut Microbiome Kits
- WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
- New Imaging Approach Could Help Predict Dangerous Gut Infection
- Rapid Sequencing Could Transform Tuberculosis Care
- Blood-Based Viral Signature Identified in Crohn’s Disease
- Hidden Gut Viruses Linked to Colorectal Cancer Risk
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
Channels
Clinical Chemistry
view channel
Blood Test Tracks Transplant Health Using Donor DNA
Organ transplantation offers life-saving treatment for patients with end-stage disease, but complications such as rejection remain a constant risk. Monitoring transplanted organs typically relies on invasive... Read more
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
Study Highlights Accuracy Gaps in Consumer Gut Microbiome Kits
Direct-to-consumer gut microbiome kits promise personalized insights by profiling fecal bacteria and generating health readouts, but their analytical accuracy remains uncertain. A new study shows that... Read more
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Agilent Technologies Acquires Pathology Diagnostics Company Biocare Medical
Agilent Technologies (Santa Clara, CA, USA) has entered into a definitive agreement to acquire Biocare Medical (Pacheco, CA, USA), expanding its pathology portfolio through the addition of highly complementary... Read more
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more