Dengue Rapid Diagnostic Tests Recycled For Serotyping
|
By LabMedica International staff writers Posted on 25 May 2016 |
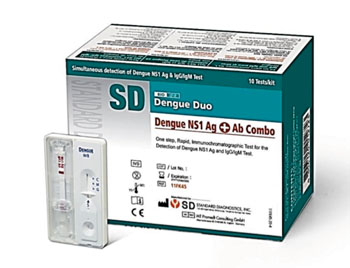
Image: The SD Bioline Dengue Duo Rapid Diagnostic Test (RDT) (Photo courtesy of Standard Diagnostics).
Dengue virus infection causes major public health problems in tropical and subtropical areas, but in many endemic areas, inadequate access to laboratory facilities is a major obstacle to surveillance and study of dengue epidemiology.
Dengue Rapid Diagnostic Tests (RDT), in which a drop of blood is loaded onto a paper strip in a plastic cassette, are simple to use and have good diagnostic accuracy. However, four types of dengue virus circulate in most tropical areas and their patterns of circulation are of epidemiological importance since they play a role in the severity and propagation of the disease.
An international team of tropical medicine specialists led by those at Mahidol University (Salaya, Thailand) collected blood samples from 99 consenting patients admitted with symptoms meeting the international criteria for dengue infection from August to November 2013. At another hospital 362 consenting patients with undifferentiated fever who tested negative by malaria RDT were enrolled from July to October 2012, and both venous whole blood and capillary whole blood from finger pricks were collected.
The samples were assayed with the SD Bioline Dengue Duo RDT (Standard Diagnostics, Kyonggi-do, Korea) which is an in vitro immunochromatographic assay for the detection of dengue virus NS1 Ag and anti-dengue IgM/IgG antibodies in human serum, plasma, or whole blood, from finger-prick or venous blood. This test comprises a pair of test devices, a dengue NS1 Ag test on the left side, and a dengue IgM/IgG antibody (Ab) test on the right side. Dengue RNA was purified from the sample pad of the NS1 RDT loaded with virus isolates of the four serotypes, then quantified by real time reverse transcriptase polymerase chain reaction (RT-PCR).
All dengue positive neat, RDT and filter paper (FP) samples were tested by real-time RT-PCR for serotyping. All four DENV serotypes were found, with the majority of patients having DENV-3 (81%; 42/52) followed by DENV-2 (10%; 5/52), DENV-4 (4%; 2/52), and DENV-1(4%; 2/52) with one sample that could not be typed. There was 100% concordance between RDT and serum RT-PCR of infecting dengue serotype. The dengue serotypes at in the rural area at Salavan were mostly DENV-1 (80%; 113/142) followed by DENV-2 (12%; 17/142) and DENV-3 (4%; 6/142).
The authors concluded that their technique may also permit dengue envelope sequencing for deeper molecular epidemiology analysis from RNA purified from RDTs. This could greatly increase availability of dengue epidemiological data from previously inaccessible tropical areas by facilitating dengue confirmation tests and strain identification to aid surveillance and public health interventions. The study was published on May 9, 2016, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
Mahidol University
Standard Diagnostics
Dengue Rapid Diagnostic Tests (RDT), in which a drop of blood is loaded onto a paper strip in a plastic cassette, are simple to use and have good diagnostic accuracy. However, four types of dengue virus circulate in most tropical areas and their patterns of circulation are of epidemiological importance since they play a role in the severity and propagation of the disease.
An international team of tropical medicine specialists led by those at Mahidol University (Salaya, Thailand) collected blood samples from 99 consenting patients admitted with symptoms meeting the international criteria for dengue infection from August to November 2013. At another hospital 362 consenting patients with undifferentiated fever who tested negative by malaria RDT were enrolled from July to October 2012, and both venous whole blood and capillary whole blood from finger pricks were collected.
The samples were assayed with the SD Bioline Dengue Duo RDT (Standard Diagnostics, Kyonggi-do, Korea) which is an in vitro immunochromatographic assay for the detection of dengue virus NS1 Ag and anti-dengue IgM/IgG antibodies in human serum, plasma, or whole blood, from finger-prick or venous blood. This test comprises a pair of test devices, a dengue NS1 Ag test on the left side, and a dengue IgM/IgG antibody (Ab) test on the right side. Dengue RNA was purified from the sample pad of the NS1 RDT loaded with virus isolates of the four serotypes, then quantified by real time reverse transcriptase polymerase chain reaction (RT-PCR).
All dengue positive neat, RDT and filter paper (FP) samples were tested by real-time RT-PCR for serotyping. All four DENV serotypes were found, with the majority of patients having DENV-3 (81%; 42/52) followed by DENV-2 (10%; 5/52), DENV-4 (4%; 2/52), and DENV-1(4%; 2/52) with one sample that could not be typed. There was 100% concordance between RDT and serum RT-PCR of infecting dengue serotype. The dengue serotypes at in the rural area at Salavan were mostly DENV-1 (80%; 113/142) followed by DENV-2 (12%; 17/142) and DENV-3 (4%; 6/142).
The authors concluded that their technique may also permit dengue envelope sequencing for deeper molecular epidemiology analysis from RNA purified from RDTs. This could greatly increase availability of dengue epidemiological data from previously inaccessible tropical areas by facilitating dengue confirmation tests and strain identification to aid surveillance and public health interventions. The study was published on May 9, 2016, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
Mahidol University
Standard Diagnostics
Latest Microbiology News
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
- Rapid Diagnostic Test Matches Gold Standard for Sepsis Detection
- Rapid POC Tuberculosis Test Provides Results Within 15 Minutes
- Rapid Assay Identifies Bloodstream Infection Pathogens Directly from Patient Samples
- Blood-Based Molecular Signatures to Enable Rapid EPTB Diagnosis
- 15-Minute Blood Test Diagnoses Life-Threatening Infections in Children
- High-Throughput Enteric Panels Detect Multiple GI Bacterial Infections from Single Stool Swab Sample
Channels
Clinical Chemistry
view channel
Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
Timely evaluation of cognitive symptoms in primary care is often limited by restricted access to specialized diagnostics and invasive confirmatory procedures. Clinicians need accessible tools to determine... Read more
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read moreMolecular Diagnostics
view channel
Molecular Monitoring Approach Helps Bladder Cancer Patients Avoid Surgery
Muscle-invasive bladder cancer is typically treated with chemotherapy followed by radical cystectomy, the complete removal of the bladder. While often effective, the surgery significantly affects quality... Read more
Genetic Tests to Speed Diagnosis of Lymphatic Disorders
Defects in the lymphatic system affect approximately one in every 3,500 newborns and can lead to severe complications, including organ failure, breathing difficulties, and life-threatening infections.... Read more
New Extraction Kit Enables Consistent, Scalable cfDNA Isolation from Multiple Biofluids
Circulating cell-free DNA (cfDNA) found in plasma, serum, urine, and cerebrospinal fluid is typically present at low concentrations and is often highly fragmented, making efficient recovery challenging... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read morePathology
view channel
Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, in part because of its dense tumor microenvironment that influences how tumors grow and respond to treatment.... Read more
New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
Sputum smear microscopy remains central to tuberculosis treatment monitoring and follow-up, particularly in high‑burden settings where serial testing is routine. Yet consistent, repeatable bacillary assessment... Read more
AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
Stem cell and bone marrow transplants can be lifesaving, but serious complications may arise months after patients leave the hospital. One of the most dangerous is chronic graft-versus-host disease, in... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreIndustry
view channel
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more