Ebola Test with Portable Reader Authorized for Emergency Use
|
By LabMedica International staff writers Posted on 26 Nov 2018 |
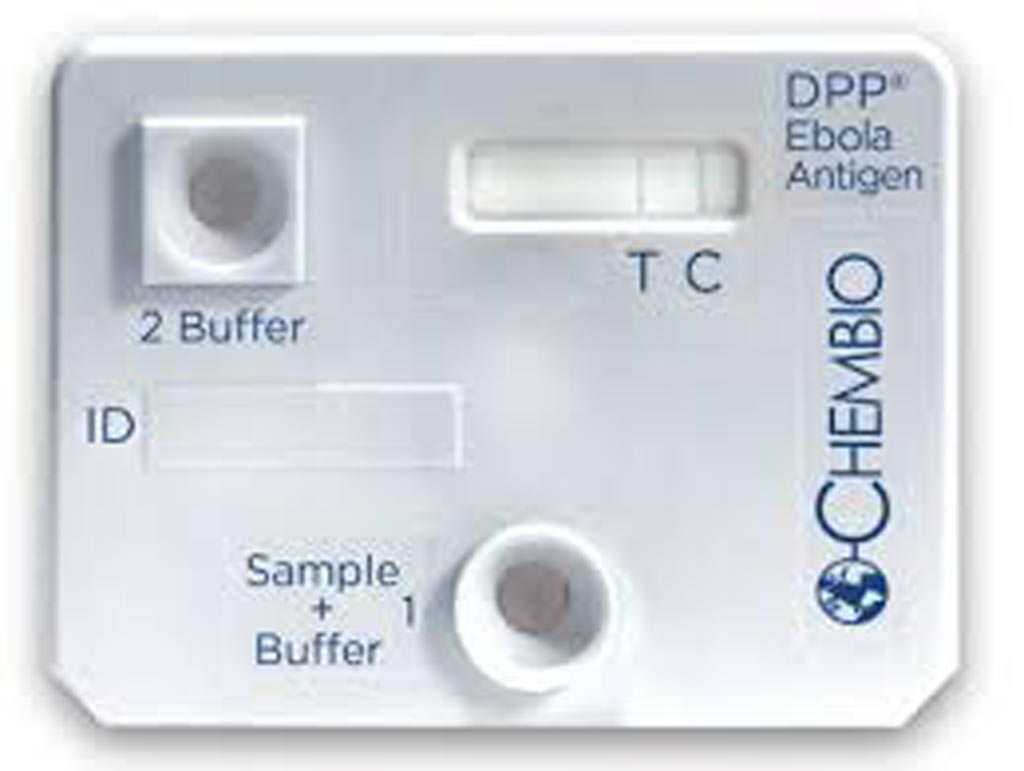
authorization (Photo courtesy of Chembio Diagnostic Systems).
Ebola is a rare but deadly virus that causes fever, body aches, and diarrhea, and sometimes bleeding inside and outside the body. As the virus spreads through the body, it damages the immune system and organs. Ultimately, it causes levels of blood-clotting cells to drop. This leads to severe, uncontrollable bleeding and kills up to 90% of people infected.
The disease is spread from human to human is through contact with blood and/or bodily fluids (urine, saliva, feces, vomit, sweat, breast milk and semen) of infected individuals. This may be through direct contact and/or droplet spread (droplets of infected bodily fluids produced by sneezing, coughing or talking) or via objects (such as needles) and environments that have been contaminated with the virus.
The US Food and Drug Administration (FDA, Silver Springs, MD, USA) issued an emergency use authorization (EUA) for a rapid, single-use test for the detection of Ebola virus (Zaire Ebola virus). This is the second Ebola rapid antigen fingerstick test available under EUA, but the first that uses a portable battery-operated reader, which can help provide clear diagnostic results outside of laboratories and in areas where patients are likely to be treated.
The test, called the DPP Ebola Antigen System (Chembio Diagnostic Systems Inc, Medford, NY, USA) is used with blood specimens, including capillary “fingerstick” whole blood, from individuals with signs and symptoms of Ebola virus disease (EVD) in addition to other risk factors, such as living in an area with large numbers of EVD cases and/or having contact with other individuals exhibiting signs and symptoms of EVD.
The DPP Ebola Antigen System provides rapid diagnostic results with tests that can be performed in locations where a healthcare provider does not have access to authorized Ebola virus nucleic acid tests (PCR testing), which are highly sensitive but can only be performed in certain laboratory settings that are adequately equipped.
The DPP Ebola Antigen System has been authorized for use with capillary “fingerstick” whole blood, ethylenediaminetetraacetic acid (EDTA, an anticoagulant added to whole blood to prevent coagulation) venous whole blood and EDTA plasma. The DPP Ebola Antigen System should only be run in facilities, including treatment centers and public health clinics where patients are likely to be treated, and laboratories that are adequately equipped, trained and capable of such testing.
It is important to note that a negative result from the DPP Ebola Antigen System, especially in patients with signs and symptoms of EVD, should not be used as the sole basis for patient management decisions. The diagnosis of EVD must be made based on multiple factors such as, history, signs, symptoms, exposure likelihood and other laboratory evidence in addition to the detection of Ebola virus.
Related Links:
US Food and Drug Administration
Chembio Diagnostic Systems
The disease is spread from human to human is through contact with blood and/or bodily fluids (urine, saliva, feces, vomit, sweat, breast milk and semen) of infected individuals. This may be through direct contact and/or droplet spread (droplets of infected bodily fluids produced by sneezing, coughing or talking) or via objects (such as needles) and environments that have been contaminated with the virus.
The US Food and Drug Administration (FDA, Silver Springs, MD, USA) issued an emergency use authorization (EUA) for a rapid, single-use test for the detection of Ebola virus (Zaire Ebola virus). This is the second Ebola rapid antigen fingerstick test available under EUA, but the first that uses a portable battery-operated reader, which can help provide clear diagnostic results outside of laboratories and in areas where patients are likely to be treated.
The test, called the DPP Ebola Antigen System (Chembio Diagnostic Systems Inc, Medford, NY, USA) is used with blood specimens, including capillary “fingerstick” whole blood, from individuals with signs and symptoms of Ebola virus disease (EVD) in addition to other risk factors, such as living in an area with large numbers of EVD cases and/or having contact with other individuals exhibiting signs and symptoms of EVD.
The DPP Ebola Antigen System provides rapid diagnostic results with tests that can be performed in locations where a healthcare provider does not have access to authorized Ebola virus nucleic acid tests (PCR testing), which are highly sensitive but can only be performed in certain laboratory settings that are adequately equipped.
The DPP Ebola Antigen System has been authorized for use with capillary “fingerstick” whole blood, ethylenediaminetetraacetic acid (EDTA, an anticoagulant added to whole blood to prevent coagulation) venous whole blood and EDTA plasma. The DPP Ebola Antigen System should only be run in facilities, including treatment centers and public health clinics where patients are likely to be treated, and laboratories that are adequately equipped, trained and capable of such testing.
It is important to note that a negative result from the DPP Ebola Antigen System, especially in patients with signs and symptoms of EVD, should not be used as the sole basis for patient management decisions. The diagnosis of EVD must be made based on multiple factors such as, history, signs, symptoms, exposure likelihood and other laboratory evidence in addition to the detection of Ebola virus.
Related Links:
US Food and Drug Administration
Chembio Diagnostic Systems
Latest Microbiology News
- Early Detection of Gut Microbiota Metabolite Linked to Atherosclerosis Could Revolutionize Diagnosis
- Viral Load Tests Can Help Predict Mpox Severity
- Gut Microbiota Analysis Enables Early and Non-Invasive Detection of Gestational Diabetes
- Credit Card-Sized Test Boosts TB Detection in HIV Hotspots
- Fecal Metabolite Profiling Predicts Mortality in Critically Ill Patients
- Portable Molecular POC System Rules Out UTIs in Just 35 Minutes
- POC Lateral Flow Test Detects Deadly Fungal Infection Faster Than Existing Techniques
- Rapid Diagnostic Test Slashes Sepsis Mortality by 39%
- Blood Culture Assay Enhances Diagnostic Stewardship Through Targeted Panel Selection
- Real-Time Genome Sequencing Detects Dangerous Superbug Causing Hospital Infections
- Diagnostic Test Accurately Detects Colorectal Cancer by Identifying Microbial Signature in Gut Bacteria
- Rapid Bedside Test Predicts Sepsis with Over 90% Accuracy
- New Blood Test Detects Up to Five Infectious Diseases at POC
- Molecular Stool Test Shows Potential for Diagnosing TB in Adults with HIV
- New Test Diagnoses Bacterial Meningitis Quickly and Accurately
- Handheld Device Delivers Low-Cost TB Results in Less Than One Hour
Channels
Clinical Chemistry
view channel
Skin Swabs Could Detect Parkinson’s Years Before Symptoms Appear
Parkinson’s disease is notoriously difficult to diagnose in its early stages, as motor symptoms do not appear until later in the progression of the disease. The ability to detect the disease up to seven... Read more
New Clinical Chemistry Analyzer Designed to Meet Growing Demands of Modern Labs
A new clinical chemistry analyzer is designed to provide outstanding performance and maximum efficiency, without compromising affordability, to meet the growing demands of modern laboratories.... Read more
New Reference Measurement Procedure Standardizes Nucleic Acid Amplification Test Results
Nucleic acid amplification tests (NAATs) play a key role in diagnosing a wide range of infectious diseases. These tests are generally known for their high sensitivity and specificity, and they can be developed... Read moreMolecular Diagnostics
view channel
Highly Accurate Biomarkers Could Detect Ovarian Cancer Before Clinical Diagnosis
Ovarian cancer is a deadly and challenging disease, primarily because early detection is difficult. Most women (70-75%) are diagnosed only after the cancer has already spread, which significantly reduces... Read more
New Gene Tool to Enable Earlier Detection and Treatment of Cardiometabolic Diseases
Cardiometabolic diseases, which affect the heart, blood vessels, and the body's ability to process food and generate energy, are difficult to diagnose early due to the complex genetic changes that contribute... Read moreHematology
view channel
Disposable Cartridge-Based Test Delivers Rapid and Accurate CBC Results
Complete Blood Count (CBC) is one of the most commonly ordered lab tests, crucial for diagnosing diseases, monitoring therapies, and conducting routine health screenings. However, more than 90% of physician... Read more
First Point-of-Care Heparin Monitoring Test Provides Results in Under 15 Minutes
Heparin dosing requires careful management to avoid both bleeding and clotting complications. In high-risk situations like extracorporeal membrane oxygenation (ECMO), mortality rates can reach about 50%,... Read moreImmunology
view channel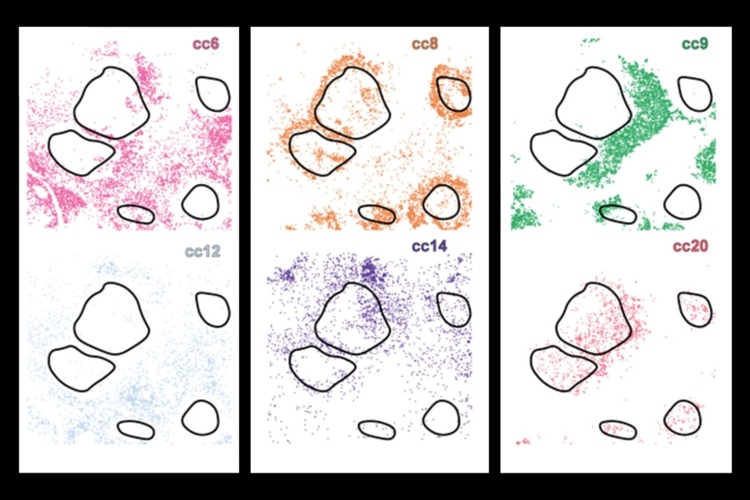
New AI System Uncovers Hidden Cell Subtypes to Advance Cancer Immunotherapy
To produce effective targeted therapies for cancer, scientists need to isolate the genetic and phenotypic characteristics of cancer cells, both within and across different tumors. These differences significantly... Read more
Evolutionary Clinical Trial to Identify Novel Biomarker-Driven Therapies for Metastatic Breast Cancer
Metastatic breast cancer, which occurs when cancer spreads from the breast to other parts of the body, is one of the most difficult cancers to treat. Nearly 90% of patients with metastatic cancer will... Read more
Groundbreaking Lateral Flow Test Quantifies Nucleosomes in Whole Venous Blood in Minutes
Diagnosing immune disruptions quickly and accurately is crucial in conditions such as sepsis, where timely intervention is critical for patient survival. Traditional testing methods can be slow, expensive,... Read morePathology
view channel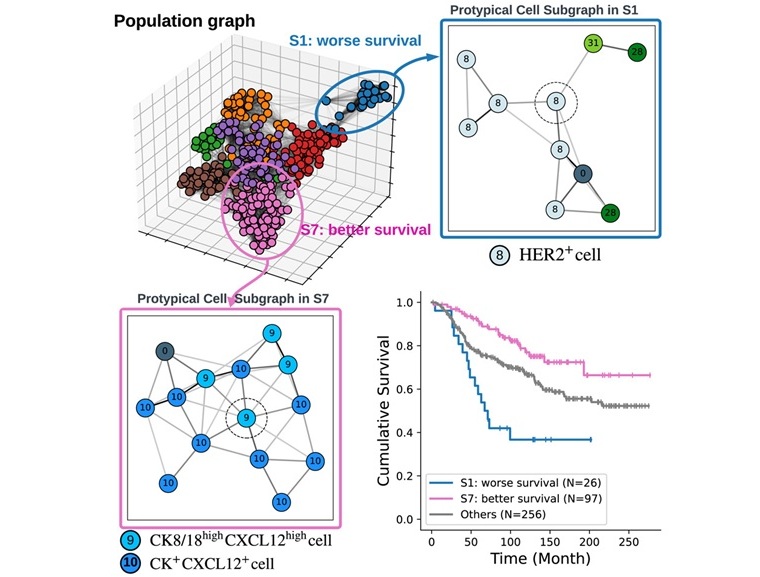
AI Tool Accurately Determines Breast Cancer Prognosis
A new study has found that cells and tissues surrounding a breast cancer tumor may hold critical information about how patients will respond to treatment. The research, published in the journal Patterns,... Read more
Powerful New Tool Improves Tissue Cancer Analysis
Studying the mix of cell types in human tissue is crucial for understanding diseases like cancer, but it presents significant challenges in both accuracy and scalability. The tumor microenvironment, composed... Read moreTechnology
view channel
Electronic Biosensors Used to Detect Pathogens Can Rapidly Detect Cancer Cells
A major challenge in healthcare is the early and affordable detection of serious diseases such as cancer. Early diagnosis remains difficult due to the complexity of identifying specific genetic markers... Read more
Safer, Portable and Low-Cost Imaging Solution to Revolutionize Biomedical Diagnostics
In diagnosing diseases and monitoring treatment, accurate and quick detection of temperature within biological tissues can be crucial, especially in early disease detection. Conventional methods such as... Read more
Multifunctional Nanomaterial Simultaneously Performs Cancer Diagnosis, Treatment, and Immune Activation
Cancer treatments, including surgery, radiation therapy, and chemotherapy, have significant limitations. These treatments not only target cancerous areas but also damage healthy tissues, causing side effects... Read moreIndustry
view channel
QuidelOrtho and BÜHLMANN Collaborate on Gastrointestinal Biomarker Tests
QuidelOrtho Corporation (San Diego, CA, USA) and BÜHLMANN Laboratories AG (Schönenbuch, Switzerland) have announced the availability of the BÜHLMANN fCAL turbo and fPELA turbo assays on QuidelOrtho's... Read more