Biomarker Based Blood Test Detects Colorectal Cancer
|
By LabMedica International staff writers Posted on 14 Apr 2015 |
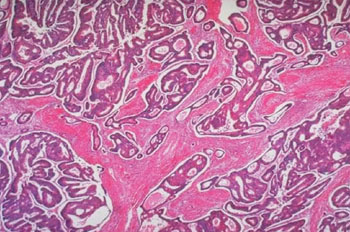
Image: Photomicrograph of a colon carcinoma shows architectural abnormalities that are even more severe than those of an adenoma (Photo courtesy of the University of Washington).
A test is needed that offers greater certainty and that can detect bowel cancer at an early stage and at the same time reaches the whole population. Should this be achieved with a blood test, it might lower the reluctance seen in patients towards the stool test.
From the moment that colorectal cancer cells are present in the body, the peripheral blood monocytes respond to the substances secreted by the cancer cells, and the immune system responds to this and tries to remove the cancer cells from the body. A specific role in this process is assigned to a specific type of white blood cell, the peripheral blood monocyte.
A large group Europeans scientists led by those at the Vesalius Research Center (VIB; Leuven, Belgium) used peripheral blood monocytes in order to characterize a distinct gene expression profile and to evaluate its potential as a candidate diagnostic biomarker in patients with colorectal cancer (CRC), a still unmet clinical need. They performed a case-control study including 360 peripheral blood monocyte samples from four European oncological centers and defined a gene expression profile specific to CRC. The robustness of the genetic profile and disease specificity were assessed in an independent setting.
The investigators found 43 putative diagnostic markers, which were refined and validated in the confirmative multicentric analysis to 23 genes with outstanding diagnostic accuracy. The diagnostic accuracy was robustly maintained in prospectively collected independent samples with a sensitivity of 92.6% and a specificity of 92.3%. This monocyte signature was expressed at early disease onset, remained robust over the course of disease progression, and was specific for the monocytic fraction of mononuclear cells. The gene modulation was induced specifically by soluble factors derived from transformed colon epithelium in comparison to normal colon or other cancer histotypes.
The expression changes were plastic and reversible, as they were abrogated upon withdrawal of tumor-released factors. Consistently, the modified set of genes reverted to normal expression upon curative treatment and was specific for CRC. The authors concluded that they are the first to demonstrate monocyte plasticity in response to tumor-released soluble factors. The identified distinct signature in tumor-educated monocytes might be used as a candidate biomarker in CRC diagnosis and harbors the potential for disease follow-up and therapeutic monitoring.
Massimiliano Mazzone, PhD, a professor and senior author of the study, said, “This study demonstrates how important it is to gain a thorough understanding of the role of our immune system in cancer. In this case, this knowledge will hopefully result in a new, more sensitive test to detect colorectal cancer at an early stage, so that more patients can be cured. I hope that we can soon find an industrial partner to help us achieve the following step, which is the development of the test.” The study was published on March 25, 2015, in the journal Gut.
Related Links:
Vesalius Research Center
From the moment that colorectal cancer cells are present in the body, the peripheral blood monocytes respond to the substances secreted by the cancer cells, and the immune system responds to this and tries to remove the cancer cells from the body. A specific role in this process is assigned to a specific type of white blood cell, the peripheral blood monocyte.
A large group Europeans scientists led by those at the Vesalius Research Center (VIB; Leuven, Belgium) used peripheral blood monocytes in order to characterize a distinct gene expression profile and to evaluate its potential as a candidate diagnostic biomarker in patients with colorectal cancer (CRC), a still unmet clinical need. They performed a case-control study including 360 peripheral blood monocyte samples from four European oncological centers and defined a gene expression profile specific to CRC. The robustness of the genetic profile and disease specificity were assessed in an independent setting.
The investigators found 43 putative diagnostic markers, which were refined and validated in the confirmative multicentric analysis to 23 genes with outstanding diagnostic accuracy. The diagnostic accuracy was robustly maintained in prospectively collected independent samples with a sensitivity of 92.6% and a specificity of 92.3%. This monocyte signature was expressed at early disease onset, remained robust over the course of disease progression, and was specific for the monocytic fraction of mononuclear cells. The gene modulation was induced specifically by soluble factors derived from transformed colon epithelium in comparison to normal colon or other cancer histotypes.
The expression changes were plastic and reversible, as they were abrogated upon withdrawal of tumor-released factors. Consistently, the modified set of genes reverted to normal expression upon curative treatment and was specific for CRC. The authors concluded that they are the first to demonstrate monocyte plasticity in response to tumor-released soluble factors. The identified distinct signature in tumor-educated monocytes might be used as a candidate biomarker in CRC diagnosis and harbors the potential for disease follow-up and therapeutic monitoring.
Massimiliano Mazzone, PhD, a professor and senior author of the study, said, “This study demonstrates how important it is to gain a thorough understanding of the role of our immune system in cancer. In this case, this knowledge will hopefully result in a new, more sensitive test to detect colorectal cancer at an early stage, so that more patients can be cured. I hope that we can soon find an industrial partner to help us achieve the following step, which is the development of the test.” The study was published on March 25, 2015, in the journal Gut.
Related Links:
Vesalius Research Center
Latest Molecular Diagnostics News
- cfDNA Testing Reduces Pregnancy Risks
- Non-Invasive Biosensor Facilitates Early Kidney Disease Detection
- New High-Sensitivity Cardiac Troponin Test Quickly Rules Out Heart Attack
- Gene Technology Outperforms Standard Newborn Screening Tests in Pioneering Study
- Maternal Blood Test Identifies Congenital Heart Diseases in Fetus
- Enhanced Ultra-Sensitive Protocol Detects Parkinson’s Disease Proteins in Extracellular Vesicles in Blood
- New RNA Molecules Can Help Predict Bowel Cancer Recurrence
- Respiratory Panel to Help Clinicians Make Precise Treatment Decisions in Outpatient Settings
- Integrating Cardiovascular Risk Biomarkers Aids in Detection of ‘Inflammaging’
- Genetic Signature in Newborns Predicts Neonatal Sepsis Before Symptoms Appear
- Integrating Multiple Protein Markers Predicts Health Outcomes in Chronic Kidney Disease Patients
- Rapid Finger Prick Blood Test to Detect Active Syphilis at Point of Care
- Urine Tests Could Reveal Early Signs of Cancer and Other Diseases
- AI-Powered Smart PCR System to Revolutionize Clinical Diagnostics
- Simple Blood Test Identifies Women in Labor at Risk for Preeclampsia
- Point-Of-Care Paper-Based Test Could Diagnose Cancer at Bedside
Channels
Clinical Chemistry
view channel.jpg)
POC Saliva Testing Device Predicts Heart Failure in 15 Minutes
Heart failure is a serious condition where the heart muscle is unable to pump sufficient oxygen-rich blood throughout the body. It ranks as a major cause of death globally and is particularly fatal for... Read more
Screening Tool Detects Multiple Health Conditions from Single Blood Drop
Infrared spectroscopy, a method using infrared light to study the molecular composition of substances, has been a foundational tool in chemistry for decades, functioning similarly to a molecular fingerprinting... Read more
Integrated Chemistry and Immunoassay Analyzer with Extensive Assay Menu Offers Flexibility, Scalability and Data Commutability
As global healthcare systems increasingly shift towards networked laboratory operational models to enhance efficiency and patient access, there is a greater need for innovative solutions tailored to the... Read moreMolecular Diagnostics
view channel
cfDNA Testing Reduces Pregnancy Risks
The highly anticipated emergence of "precision medicine" promises customized technologies that can benefit individuals while potentially lowering healthcare costs. Now, new research suggests that pregnancy... Read more
Non-Invasive Biosensor Facilitates Early Kidney Disease Detection
Traditionally, kidney function has been assessed by measuring blood creatinine levels, which reflect muscle breakdown. Elevated creatinine levels may indicate that the kidneys are not effectively filtering waste.... Read moreHematology
view channel
Next Gen CBC and Sepsis Diagnostic System Targets Faster, Earlier, Easier Results
Every hour is critical in protecting patients from infections, yet there are currently limited tools to assist in early diagnosis before patients reach a hospital. The complete blood count (CBC) is a common... Read more
Newly Discovered Blood Group System to Help Identify and Treat Rare Patients
The AnWj blood group antigen, a surface marker discovered in 1972, has remained a mystery regarding its genetic origin—until now. The most common cause of being AnWj-negative is linked to hematological... Read more
Blood Platelet Score Detects Previously Unmeasured Risk of Heart Attack and Stroke
Platelets, which are cell fragments circulating in the blood, play a critical role in clot formation to stop bleeding. However, in some individuals, platelets can become "hyperreactive," leading to excessive... Read moreImmunology
view channel.jpg)
Advanced Imaging Method Maps Immune Cell Connections to Predict Cancer Patients Survival
A growing tumor is influenced not only by the tumor cells themselves but also by the surrounding tissue, which alters its biology. Immune cells communicate by transferring vital signaling proteins to their... Read more
Computational Tool Predicts Immunotherapy Outcomes for Metastatic Breast Cancer Patients
Immunotherapy aims to enhance the body’s immune response to target cancer cells, but not all patients experience a positive reaction to such treatments. Identifying which patients will benefit from immunotherapy... Read more
Biomarker Could Predict Immunotherapy Response in Liver Cancer
Until recently, patients diagnosed with hepatocellular carcinoma had limited treatment options, with existing therapies extending life by only a few months. Immunotherapy has emerged as a new alternative... Read more
Epigenetic Test Could Determine Efficacy of New Immunotherapy Treatments Against Multiple Myeloma
Multiple myeloma is a blood cancer that primarily affects individuals over the age of sixty, and its occurrence rises as the population ages. In this disease, the bone marrow—the spongy tissue inside bones... Read moreMicrobiology
view channel
High-Accuracy Bedside Test to Diagnose Periprosthetic Joint Infection in Five Minutes
Periprosthetic joint infection (PJI) represents a significant global issue that is worsening as the number of joint replacements increases due to aging populations. In the United States alone, the anticipated... Read more_1.jpg)
Innovative Diagnostic Approach for Bacterial Infections to Enable Faster and Effective Treatment
For patients with bacterial infections, timely treatment with the appropriate antibiotics significantly improves their chances of recovery. Current methods for identifying which antibiotics will be effective... Read more
Non-Invasive Stool Test to Diagnose Endometriosis and Help Reduce Disease Progression
Endometriosis, a painful condition impacting nearly 200 million women globally, occurs when tissue similar to the lining of the uterus grows outside its usual location, such as on the intestines or the... Read more
Automated Positive Blood Culture Sample Preparation Platform Designed to Fight Against Sepsis and AMR
Delayed administration of antibiotics to patients with bloodstream infections significantly increases the risk of morbidity and mortality. For optimal therapeutic outcomes, it is crucial to rapidly identify... Read moreTechnology
view channel
New Noninvasive Methods Detect Lead Exposure Faster, Easier and More Accurately at POC
Exposure to lead can negatively affect health in multiple ways, leading to damage in the brain and central nervous system, delays in development and growth, learning and behavioral issues, problems with... Read more
Noninvasive Test Detects Malaria Without Blood Sample
Malaria remains a significant global health issue, with approximately 250 million cases and over 600,000 deaths reported annually. Nearly half of the world's population is at risk for malaria infection,... Read moreIndustry
view channel
Microbiologics Acquires Diagnostic Quality Controls Manufacturer SensID
Microbiologics (St. Cloud, MN, USA), a biotechnology company specializing in infectious disease reference materials and contract research services, has acquired SensID (Rostock, Germany), a manufacturer... Read more














_1.jpg)