Noninvasive Test Detects Malaria Without Blood Sample
Posted on 30 Oct 2024
Malaria remains a significant global health issue, with approximately 250 million cases and over 600,000 deaths reported annually. Nearly half of the world's population is at risk for malaria infection, particularly vulnerable groups such as children and pregnant women, who face the highest likelihood of severe illness and death from the disease. Currently, detecting this potentially fatal infection typically requires invasive blood samples, and existing testing methods have considerable limitations that hamper their effectiveness. A new technology now presents an exciting point-of-care (POC) diagnostic tool that has the potential to improve malaria detection and facilitate timely treatment.
Researchers at Yale School of Public Health (New Haven, CT, USA) and their collaborators have introduced a novel noninvasive test that could significantly transform malaria testing in low- and middle-income countries that are heavily impacted by this mosquito-borne illness. The innovative test does not require any blood samples, making it safer and more accessible. It utilizes a device called the Cytophone, which employs targeted lasers and ultrasound to identify malaria-infected cells in the bloodstream. Roughly the size of a tabletop printer, the Cytophone prototype can quickly ascertain the presence of malaria infection through a small, noninvasive probe applied to the back of a patient’s hand over a targeted vein.
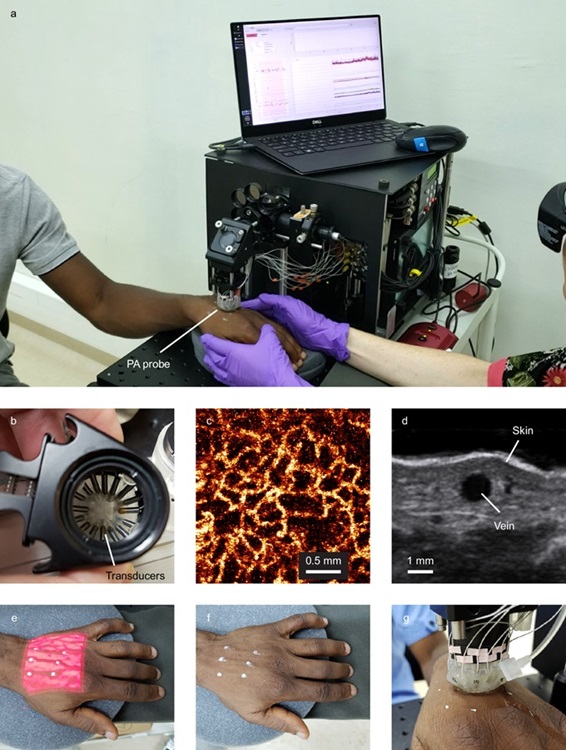
The Cytophone's ability to detect infections noninvasively is made possible due to the accumulation of a by-product known as hemozoin in red blood cells infected with malaria parasites. This iron crystal by-product absorbs more light than normal hemoglobin when exposed to laser light, heating up and displaying magnetic and optical properties that the Cytophone probe can identify. In research published in Nature Communications, the team evaluated the Cytophone on 20 adult patients diagnosed with symptomatic malaria in Cameroon. The device successfully identified Plasmodium falciparum, the most prevalent and lethal malaria parasite, along with other less common species. The findings demonstrated that the Cytophone is sensitive enough to detect both high and low levels of parasites in infected blood, achieving 90% sensitivity and 69% specificity—comparable to, and in some cases exceeding, current gold standards for malaria testing that necessitate blood draws. Additionally, the device was capable of tracking the reduction of parasite levels when patients were retested post-treatment.
“Our study showed that the Cytophone was safe and had comparable diagnostic performance to current point-of-care options when compared to highly sensitive quantitative PCR as the gold standard,” said Jillian N. Armstrong, one of the study’s lead authors.













