Mobile DNA Test Developed for HIV
|
By LabMedica International staff writers Posted on 19 Jun 2014 |
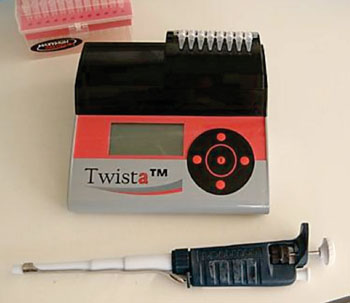
Image: The Twista portable real-time fluorometer (Photo courtesy of TwistDx Ltd).
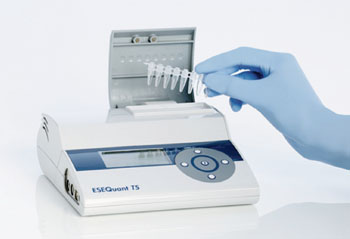
Image: ESEQuant Tube Scanner for the measurement of fluorescence in tubes in point-of-need applications (Photo courtesy of Qiagen).
An efficient test to detect signs of human immunodeficiency virus (HIV) and its progress in patients in low-resource settings is being developed using recombinase polymerase amplification (RPA).
The current gold standard to diagnose HIV in infants and to monitor viral load depends on laboratory equipment and technical expertise generally available only in clinics, while the new assay features a nucleic acid-based test that can be performed at the site of care.
Bioengineers at Rice University (Houston, TX, USA) developed a new technique that would replace a complex procedure based on polymerase chain reaction (PCR) with one that relies on RPA, a method that quickly amplifies genetic markers found in blood to levels where they can be easily counted. In the test the team calls quantitative RPA (qRPA), a specific sequence in HIV DNA is targeted and tagged with fluorescent probes that can be seen and quantified by a portable machine. Software analysis of the fluorescing DNA allows clinicians to determine with great accuracy whether the virus is present in a patient's blood and/or how much is there.
Amplification and real-time data collection were performed in a CFX96 Real Time qPCR machine (Bio-Rad; Hercules, CA, USA). Preliminary RPA experiments detecting HIV-1 DNA and internal positive control sequence (IPC) DNA in separate reactions demonstrated that the time at which detectable amplification begins, increases with decreasing DNA concentration, suggesting that quantification of DNA with RPA is feasible. The assay could potentially be optimized for greater accuracy by collecting fluorescence data more frequently or decreasing the reaction rate by either decreasing the concentration of magnesium acetate in the reaction or amplifying at a lower temperature.
This assay was designed for use with an inexpensive, point-of-care fluorescence reader, such as the commercially available Twista portable real-time fluorometer (TwistDx, Ltd.; Cambridge, UK) or the ESEQuant Tube Scanner (Qiagen; Valencia, CA, USA). To be clinically viable, a DNA-based test for HIV has to be able to quantify virus loads over four orders of magnitude, from very low to very high; the scientists reported that the qRPA test easily meets that goal. The study was published on May 26, 2014, in the journal Analytical Chemistry.
Related Links:
Rice University
Bio-Rad
TwistDx Ltd.
The current gold standard to diagnose HIV in infants and to monitor viral load depends on laboratory equipment and technical expertise generally available only in clinics, while the new assay features a nucleic acid-based test that can be performed at the site of care.
Bioengineers at Rice University (Houston, TX, USA) developed a new technique that would replace a complex procedure based on polymerase chain reaction (PCR) with one that relies on RPA, a method that quickly amplifies genetic markers found in blood to levels where they can be easily counted. In the test the team calls quantitative RPA (qRPA), a specific sequence in HIV DNA is targeted and tagged with fluorescent probes that can be seen and quantified by a portable machine. Software analysis of the fluorescing DNA allows clinicians to determine with great accuracy whether the virus is present in a patient's blood and/or how much is there.
Amplification and real-time data collection were performed in a CFX96 Real Time qPCR machine (Bio-Rad; Hercules, CA, USA). Preliminary RPA experiments detecting HIV-1 DNA and internal positive control sequence (IPC) DNA in separate reactions demonstrated that the time at which detectable amplification begins, increases with decreasing DNA concentration, suggesting that quantification of DNA with RPA is feasible. The assay could potentially be optimized for greater accuracy by collecting fluorescence data more frequently or decreasing the reaction rate by either decreasing the concentration of magnesium acetate in the reaction or amplifying at a lower temperature.
This assay was designed for use with an inexpensive, point-of-care fluorescence reader, such as the commercially available Twista portable real-time fluorometer (TwistDx, Ltd.; Cambridge, UK) or the ESEQuant Tube Scanner (Qiagen; Valencia, CA, USA). To be clinically viable, a DNA-based test for HIV has to be able to quantify virus loads over four orders of magnitude, from very low to very high; the scientists reported that the qRPA test easily meets that goal. The study was published on May 26, 2014, in the journal Analytical Chemistry.
Related Links:
Rice University
Bio-Rad
TwistDx Ltd.
Latest Technology News
- New Diagnostic System Achieves PCR Testing Accuracy
- DNA Biosensor Enables Early Diagnosis of Cervical Cancer
- Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
- Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
- First of Its Kind Technology Detects Glucose in Human Saliva
- Electrochemical Device Identifies People at Higher Risk for Osteoporosis Using Single Blood Drop
- Novel Noninvasive Test Detects Malaria Infection without Blood Sample
- Portable Optofluidic Sensing Devices Could Simultaneously Perform Variety of Medical Tests
- Point-of-Care Software Solution Helps Manage Disparate POCT Scenarios across Patient Testing Locations
- Electronic Biosensor Detects Biomarkers in Whole Blood Samples without Addition of Reagents
- Breakthrough Test Detects Biological Markers Related to Wider Variety of Cancers
- Rapid POC Sensing Kit to Determine Gut Health from Blood Serum and Stool Samples
- Device Converts Smartphone into Fluorescence Microscope for Just USD 50
- Wi-Fi Enabled Handheld Tube Reader Designed for Easy Portability
Channels
Clinical Chemistry
view channel
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
Novel Biomarkers to Improve Diagnosis of Renal Cell Carcinoma Subtypes
Renal cell carcinomas (RCCs) are notably diverse, encompassing over 20 distinct subtypes and generally categorized into clear cell and non-clear cell types; around 20% of all RCCs fall into the non-clear... Read more
RNA-Powered Molecular Test to Help Combat Early-Age Onset Colorectal Cancer
Colorectal cancer (CRC) ranks as the second most lethal cancer in the United States. Nevertheless, many Americans eligible for screening do not undergo testing due to limited access or reluctance towards... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read morePathology
view channelHyperspectral Dark-Field Microscopy Enables Rapid and Accurate Identification of Cancerous Tissues
Breast cancer remains a major cause of cancer-related mortality among women. Breast-conserving surgery (BCS), also known as lumpectomy, is the removal of the cancerous lump and a small margin of surrounding tissue.... Read more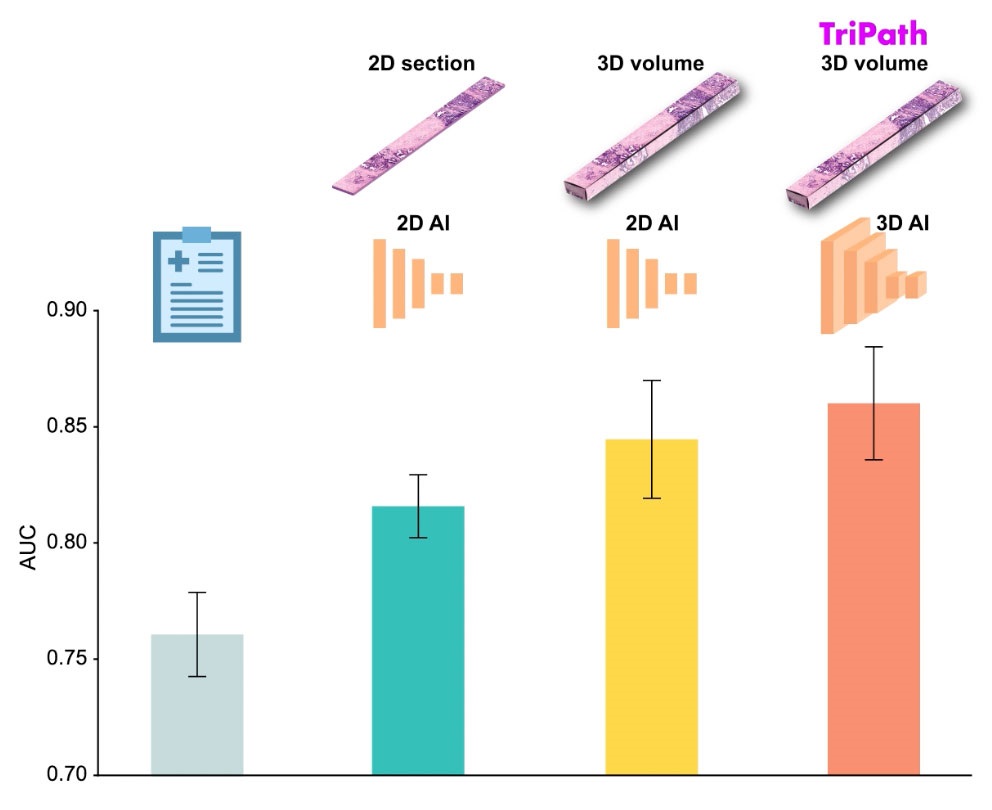
AI Advancements Enable Leap into 3D Pathology
Human tissue is complex, intricate, and naturally three-dimensional. However, the thin two-dimensional tissue slices commonly used by pathologists to diagnose diseases provide only a limited view of the... Read more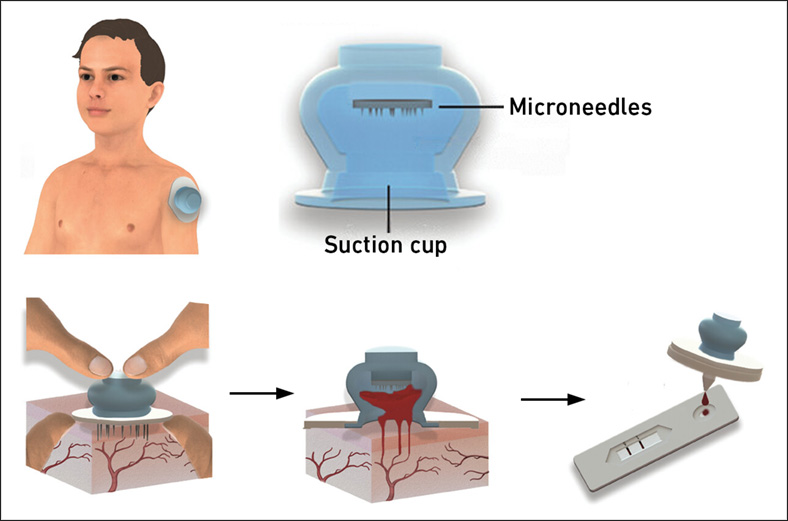
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more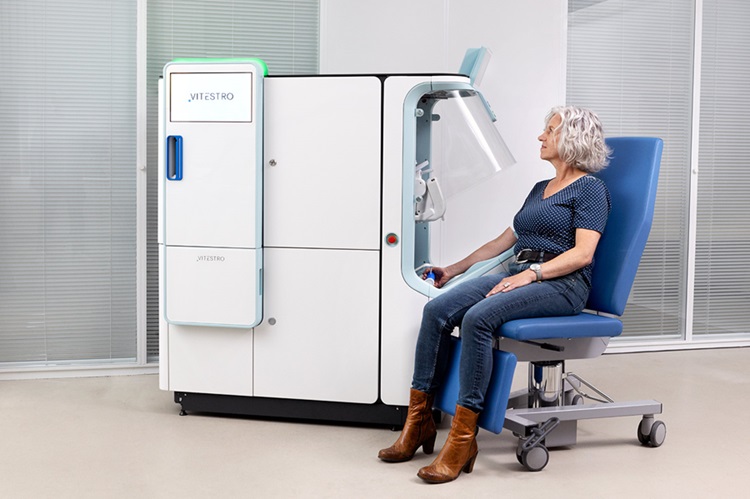
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more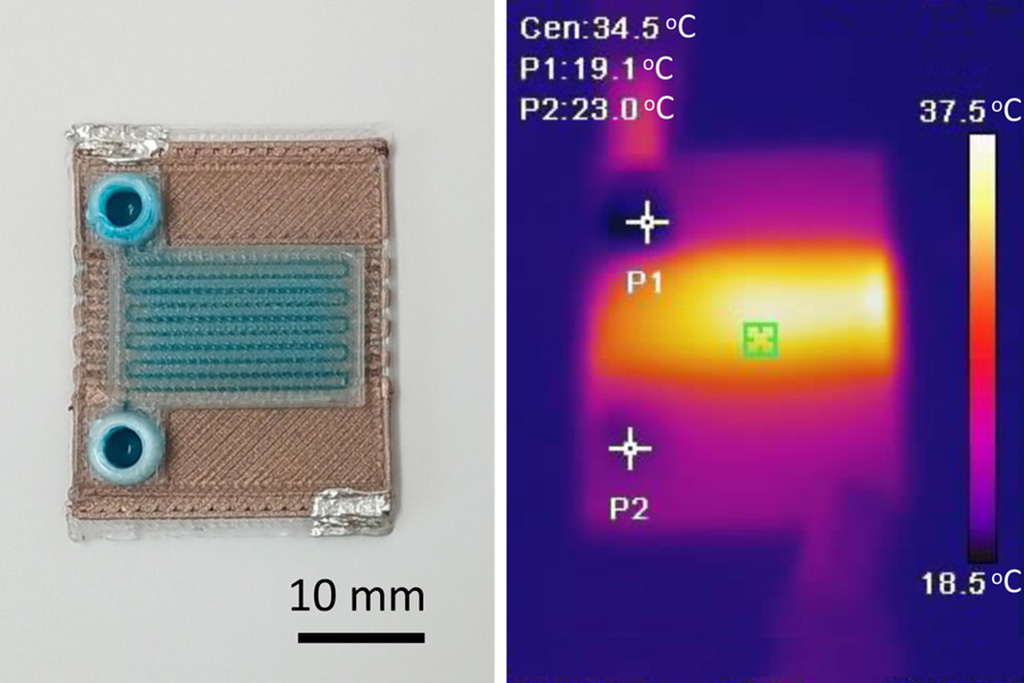
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
Unlike severe traumatic brain injury (TBI), mild TBI often does not show clear correlations with abnormalities detected through head computed tomography (CT) scans. Consequently, there is a pressing need... Read more
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)