Molecular Diagnostics
Biomarker Test Detects Alzheimer’s Neurodegeneration in Blood
Scientists have applied their knowledge of molecular biology and biochemistry of tau proteins in different tissues, such as the brain, to develop a technique to selectively detect “brain-derived tau,” or BD-tau, a novel marker of Alzheimer’s disease neurodegeneration, while avoiding free-floating “big tau” proteins produced by cells outside the brain. More...28 Dec 2022

Tuberculosis Pathogen Dynamics Detected With Nanopore Sequencing
Mycobacterium tuberculosis whole-genome sequencing (WGS) has been widely used for genotypic drug susceptibility testing (DST) and outbreak investigation. For both applications, Illumina technology is used by most public health laboratories. More...26 Dec 2022
Real-Time PCR-Based Serological Diagnosis of Human Granulocytic Anaplasmosis
Human granulocytic anaplasmosis (HGA) is a tick-borne infection caused by the intracellular bacterium Anaplasma phagocytophilum, an emerging pathogen. HGA can manifest as a subclinical infection; however, most symptomatic persons have fever, myalgia, and headache associated with thrombocytopenia, leukopenia, and elevated transaminase levels. More...26 Dec 2022
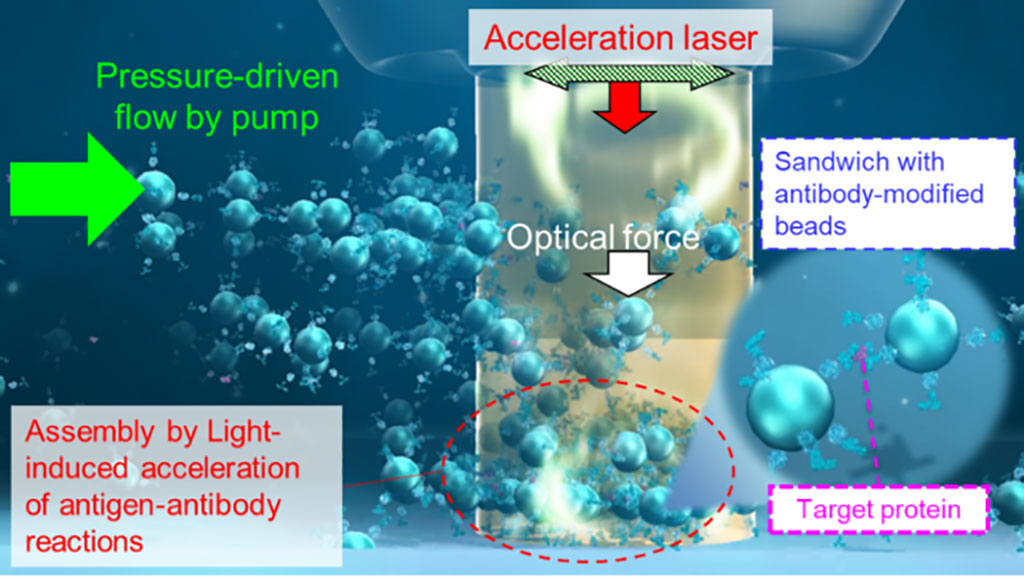
Ultrafast, Ultra-Sensitive Protein Detection Method Enables Ultra-Early Disease Diagnosis
Methods to analyze trace amounts of proteins based on antigen–antibody reaction enable diagnosis at an early stage of many diseases, including cancer, dementia, and microbial infections. Now, a team of scientists has discovered a new principle underlying light-induced acceleration of antigen–antibody reaction, allowing for simple, ultrafast, and highly sensitive detection of proteins. More...23 Dec 2022

Extremely Sensitive Fluorescence Lateral Flow Reader Detects Even Low Positives Results
Presently, fluorescence lateral flow tests represent an affordable alternative to the PCR tests guaranteeing a high sensitivity and specificity. Now, an off-the-shelf lateral flow reader designed to scan fluorescence-labeled lateral flow tests represents a fundamental advance in this field. More...22 Dec 2022
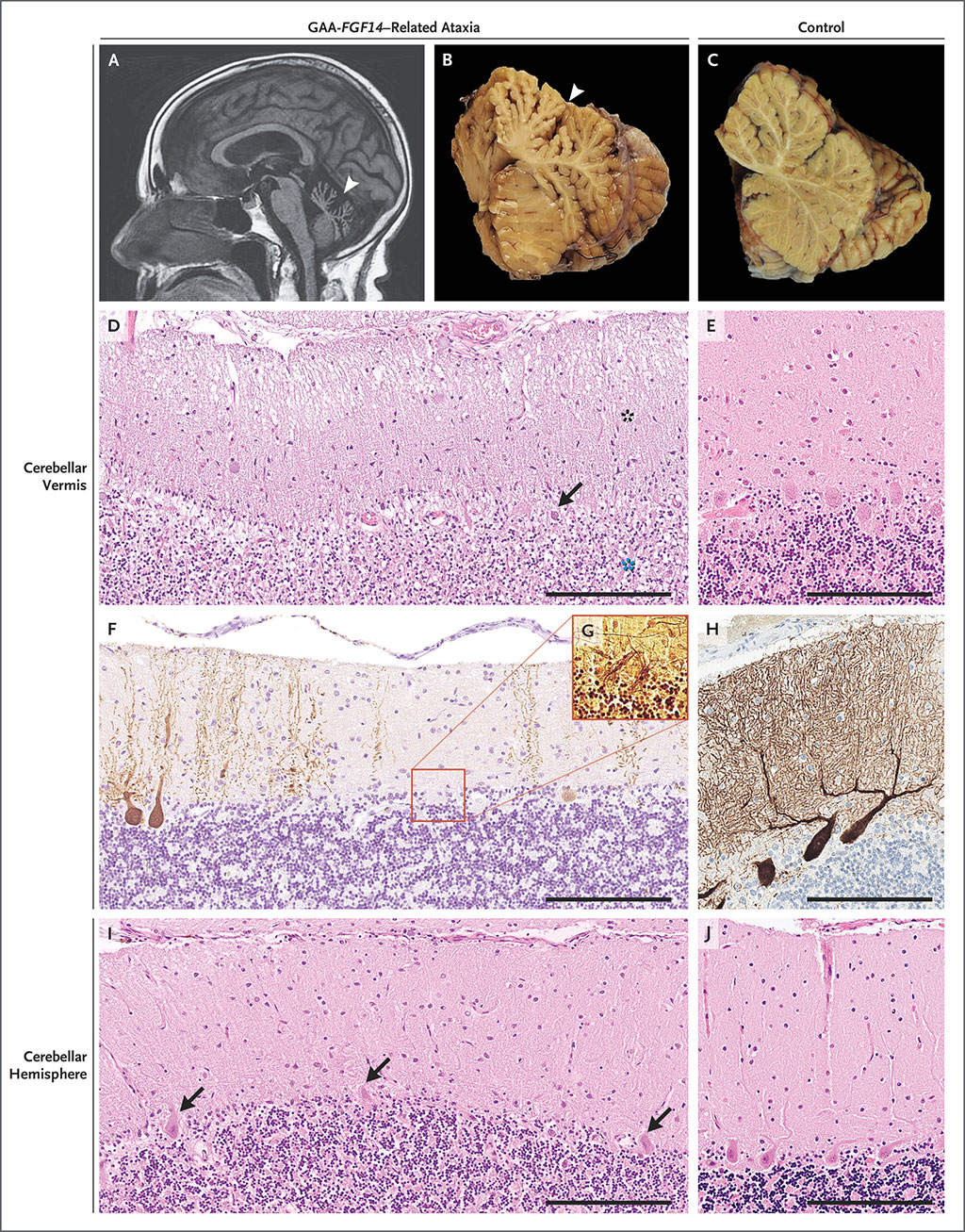
Late-Onset Ataxia Linked to Repeat Expansion in FGF14 Gene
There are many causes of cerebellar ataxia including, among others, gluten ataxia, autoimmunity to Purkinje cells or other neural cells in the cerebellum, CNS vasculitis, multiple sclerosis, infection, bleeding, infarction, tumors, direct injury, toxins, genetic disorders and neurodegenerative diseases (such as progressive supranuclear palsy and multiple system atrophy). More...20 Dec 2022
In Other News
POC Assay Could Diagnose Acute Coronary Syndrome in 3-5 Minutes
PCR Test Detects Non-Variola Orthopoxviruses, Including Monkeypox Virus, in 3.5 Hours
POC Molecular TB Test Could Enable Clinicians to Begin Appropriate Treatment Immediately
New Digital Platform Technology Aims to Disrupt 50-Year-Old PCR Testing for Similar-Symptom Pathogens
Lupus Heterogeneity Highlighted With Single-Cell Transcriptomes
PCR-Based CRC Early Detection Stool Test Fills Important Gap Between FIT and Colonoscopy
Early-Pregnancy Urine Test Could Predict Preeclampsia
Portable qPCR Platform Enables Faster, Better, Low-Cost Near-Patient Molecular Diagnosis
Long COVID Etiologies Found in Acute Infection Blood Samples
POCT Immunoassay Reader Provides Quantitative Analysis of Test Kits for More Accurate Diagnosis
World’s First Rapid Saliva-Based Pregnancy Test Eliminates Need for Blood and Urine Samples
Cepheid's Multi-Target Test Design Enables Detection of Influenza Variants
Portable UTI Molecular Diagnostic Platform Provides Accurate Test Results in 40 Minutes
New Blood Test Detects ‘Toxic’ Protein Years Before Alzheimer’s Symptoms Emerge
Biomarkers Predict Lyme Disease Post-Treatment Prognosis
Low Cost, Highly Sensitive Blood Test for Cancer Makes Regular Monitoring Affordable
New All-In-One Molecular Diagnostic System Realizes True Sample In -Result Out Detection Process
Method Enables Targeted Profiling of Human Extrachromosomal DNA
ctDNA-Based MRD Test to Detect As Few As One Mutant Molecule in a Million DNA Molecules
Study Identifies Optimal Blood Tests for Detection and Treatment of Early Stage Alzheimer’s
Blood Test for Protein Biomarker Could Detect Pancreatic Cancer at Very Early Stage
Blood Tests Could Predict Survival Odds for Patients with Metastatic Cancer
New Serological Test Could Search for Viral Triggers of Diseases
Genetic Testing channel of LabMedica brings the latest in molecular genetics, cytogenetics, and epigenetics, and methods from PCR to FISH, and more.










