
Quest Diagnostics’ SARS-CoV-2 rRT-PCR Test Becomes First COVID-19 Diagnostic Test to Receive FDA EUA for Sample Pooling
The US Food and Drug Administration has reissued an emergency use authorization (EUA) to Quest Diagnostics (Secaucus, NJ, USA) to authorize its Quest SARS-CoV-2 rRT-PCR test for use with pooled samples. More...20 Jul 2020
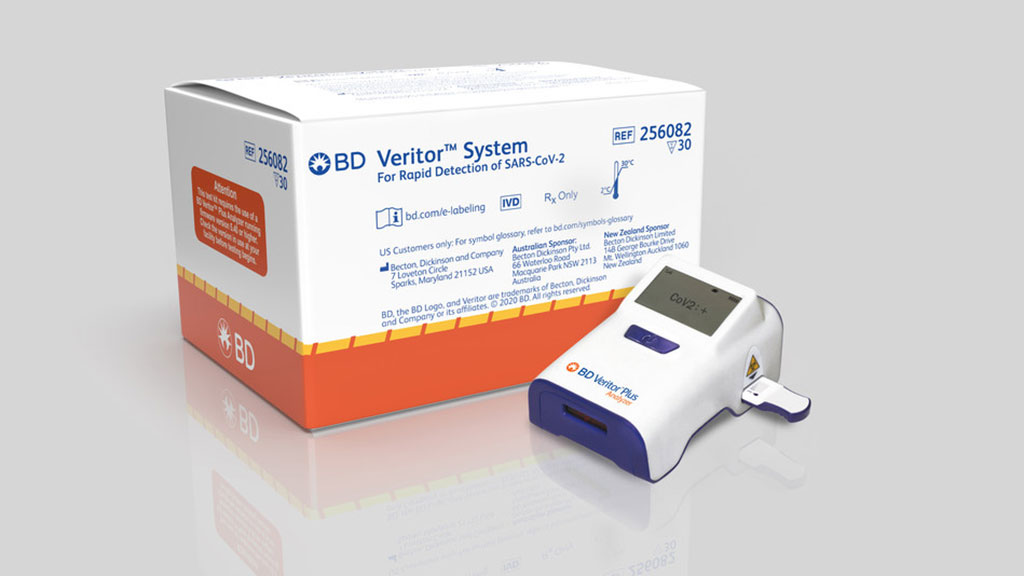
BD’s Point-of-Care COVID-19 Tests and Instruments to Help US Government Increase Testing Capacity
The US Department of Health and Human Services (HHS) has committed to purchase 2,000 BD Veritor Plus Systems and 750,000 SARS-CoV-2 antigen test kits for use on the system from Becton, Dickinson and Company (BD Franklin Lakes, NJ, USA). More...16 Jul 2020
First-in-Class COVID-19 Rapid Diagnostic Test for Neutralizing Antibodies Could Serve as Immunity Passport for Vaccine Developers
A new rapid diagnostic test for measuring levels of functional neutralizing antibodies that are believed to prevent SARS-CoV-2 from entering the host cells could serve as an ‘Immunity Passport’ for vaccine developers around the world as they begin larger Phase II and III clinical trials. More...16 Jul 2020
Metagenomics to Help Develop Targeted Respiratory Pathogen Panel for Identifying SARS-CoV-2 and 250 Other Pathogens
Metagenomics analysis is transforming highly sophisticated research-only laboratory tools into practical diagnostics tools for routine hospital use, such as allowing physicians to determine whether a patient has COVID-19 and use the same test to confirm whether that patient has other respiratory pathogens exacerbating the illness. More...16 Jul 2020

In Other News
EKF Diagnostics Launches Novel Molecular Transport Media for Dual COVID-19 and Influenza Sampling
Enzo Biochem Secures FDA Emergency Use Authorization for Proprietary Coronavirus Detection Test System
New SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit Enables Visual Detection of Coronavirus in 30 Minutes
Diazyme Receives FDA Emergency Use Authorization for New COVID-19 Antibody Test
EpiGentek Launches First-to-Market Kits to Detect SARS-CoV-2-Targeted Furin Activity and Screen for Inhibitors Against COVID-19
Triple Antibody Test for COVID-19 Provides Laboratory-Standard Results in Just Seven Minutes
New Rapid 45-Minute COVID-19 Test Ideal for Screening at Points of Risk
Quantabio RT-qPCR Kit Given Expanded Role in CDC COVID-19 Testing Protocol
Diasorin CE Marks New Simplexa Flu A/B & RSV Direct Gen II Assay to Run with Simplexa COVID-19 Direct Assay
Advanced Biological Releases First Commercial CE-IVD Registered COVID-19 rRT-PCR Assay Also Validated for Saliva
BD Launches Portable, Rapid POC Antigen Test to Detect SARS-CoV-2 in 15 Minutes
COVID-19-Influenza Combination Test Granted FDA Emergency Use Authorization
Transasia Launches COVID-19 IgG Antibody Kits with 98% Accuracy and at 20% Cost of RT-PCR Tests
Erba Mannheim Makes SARS-CoV-2 Testing Available to Labs Everywhere
Fluxergy Collaborates with Mass General Brigham to Evaluate One-Hour COVID-19 Diagnostic Test
Eurofins Launches CE-IVD Marked Rapid POC Testing Devices to Identify Past Exposure to COVID-19 in 10 Minutes
T2 Biosystems Launches New COVID-19 Molecular Diagnostic Test
Roche Secures FDA Emergency Use Authorization for Elecsys IL-6 Test to Identify Severe Inflammatory Response in COVID-19 Patients
Beckman Coulter's SARS-CoV-2 IgG Antibody Test Granted FDA Emergency Use Authorization
Blood Test Can Predict Severity of COVID-19
New One-Piece Injection-Molded COVID-19 Swab to Enable Expanded Testing
Experts Identify Steps to Expand and Improve Antibody Tests in COVID-19 Response
New Platform to Accelerate COVID-19 Tests into Real-World Use











